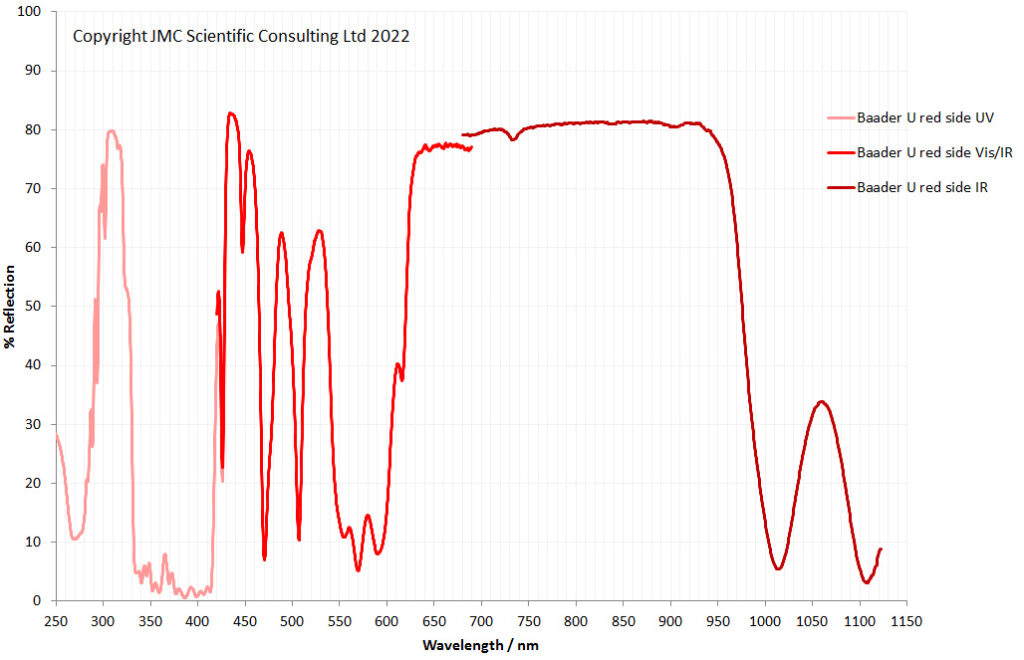
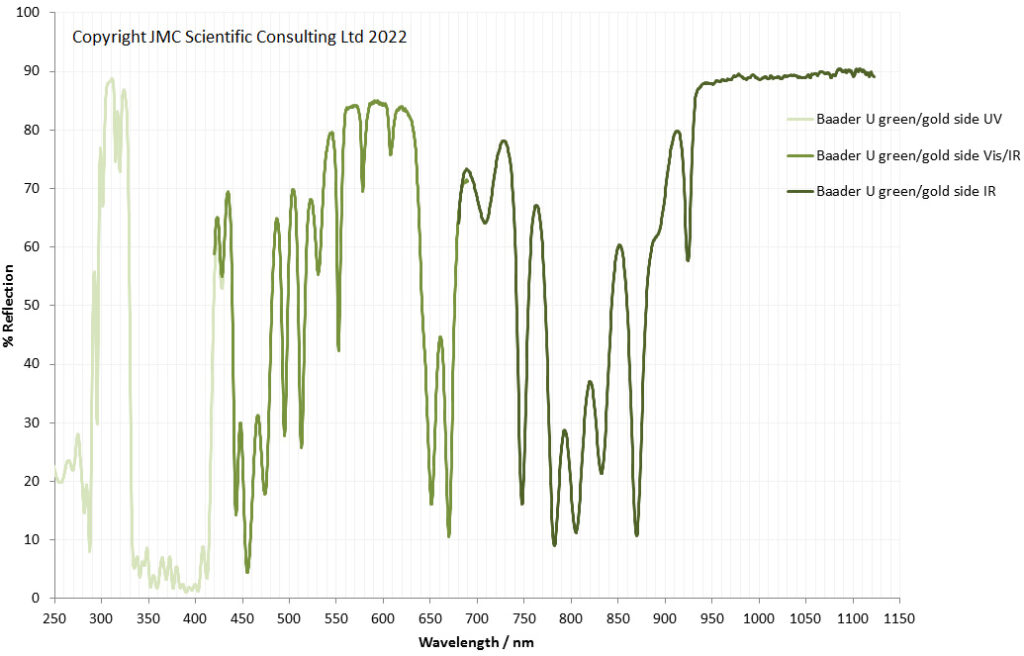
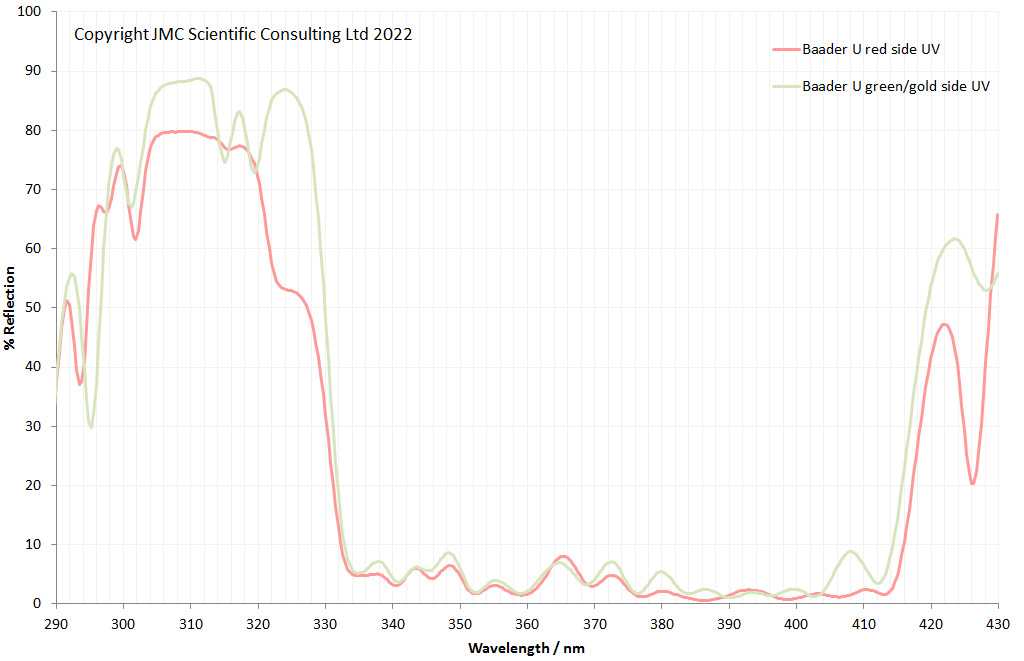
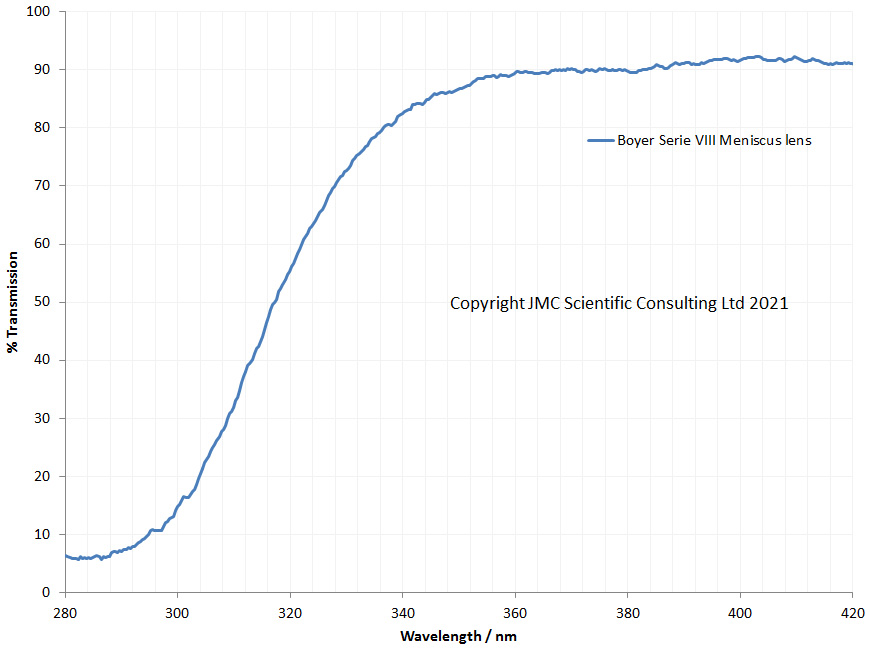
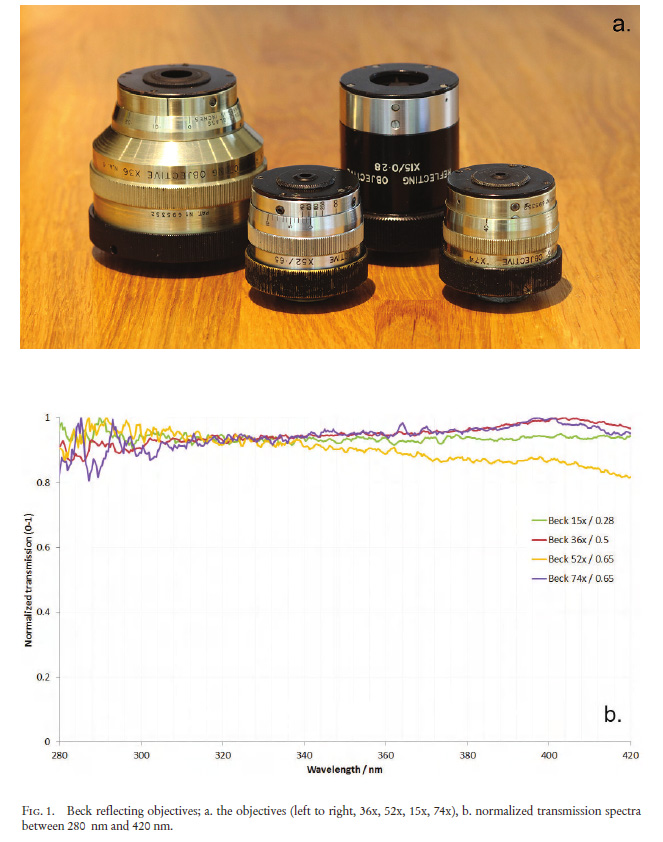
“Few objects are more beautiful than the minute siliceous cases of the diatomaceae: were these created that they might be examined and admired under the higher powers of the microscope? The beauty in this latter case, and in many others, is apparently wholly due to symmetry of growth…”, Charles Darwin, On the Origin of Species, 1866.
While I spend a lot of my time doing research for other people, I am also a simple scientist at heart. Like many people I appreciate beauty in art, but for me, I tend to seek patterns and structure in what I am looking at. Something that gives me both beauty and structure is looking at diatoms under the microscope. These are the solid structures left behind when certain species of algae die off and they are beautiful structures. I’ve not graduated to making my own microscope slides just yet, so tend to keep an eye out for second hand ones on eBay. Some can be had for a few GBP (although for big arrangements you can pay thousands of GBP, especially from certain makers as there is a big collectors market) and they make for fascinating viewing under the microscope at the end of a long week – microscope therapy as it were. Today I want to share with you some images from a couple of slides which arrived this week.
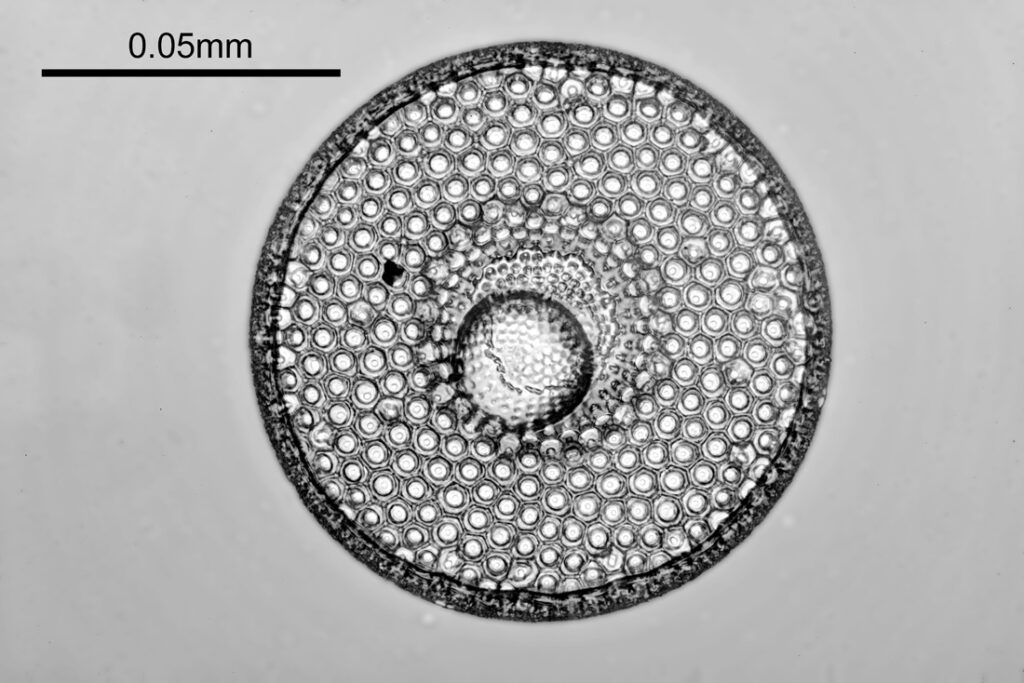
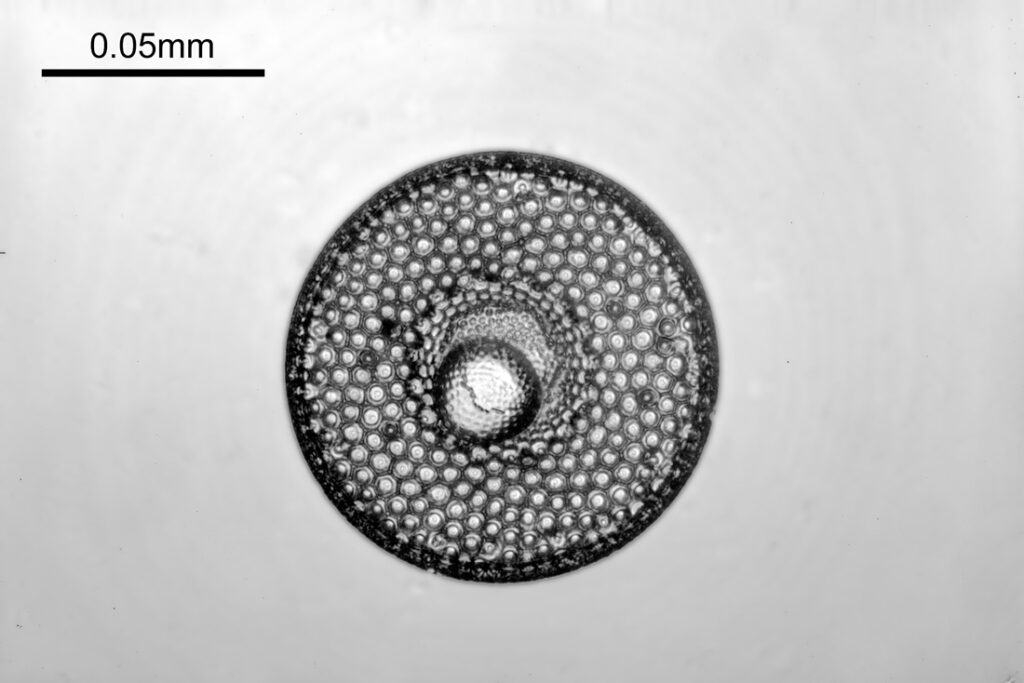
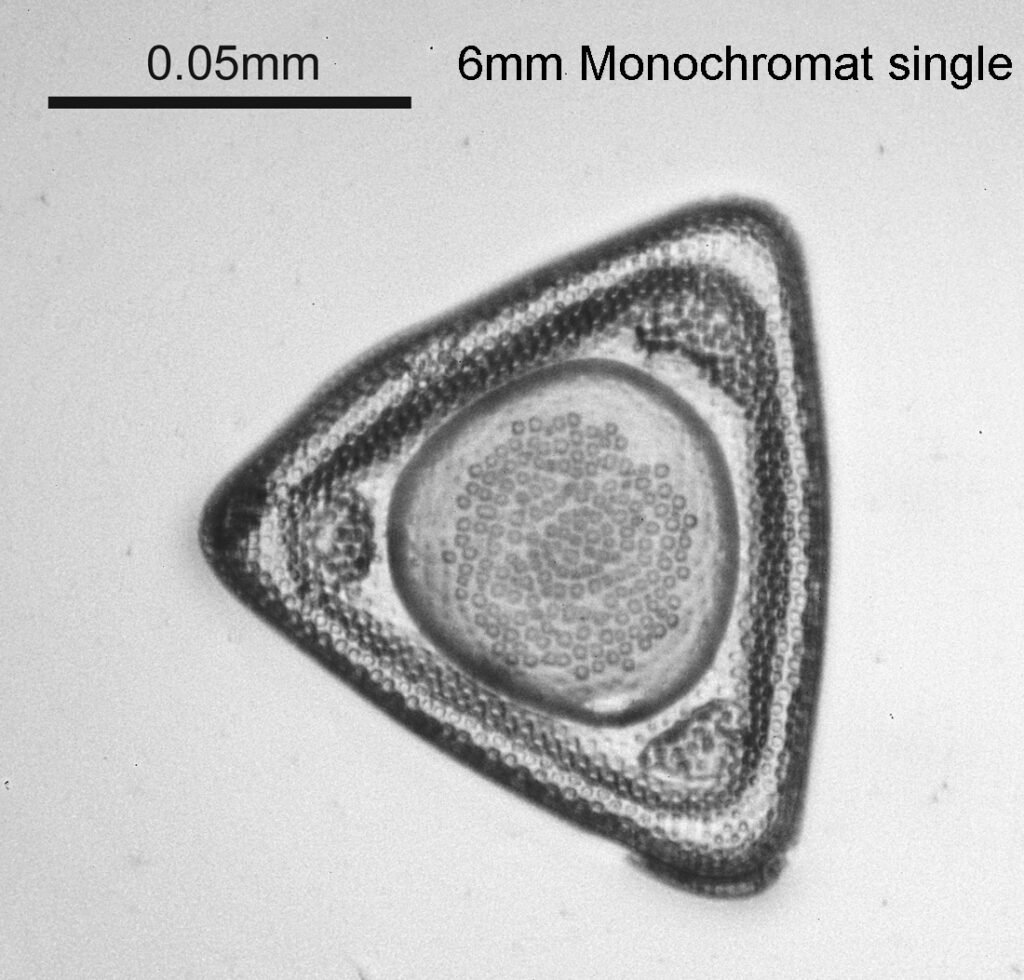
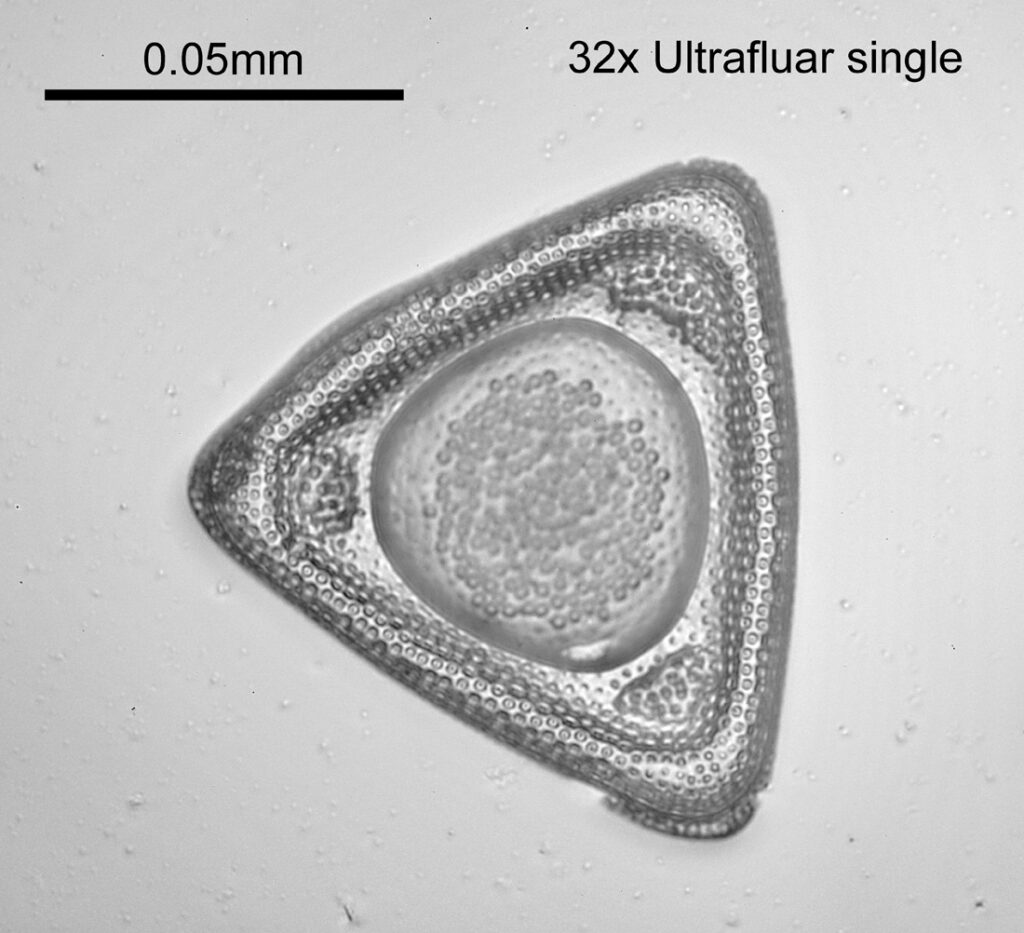
Two slides arrived this week – one is an antique slide made by John Barnett which has an arrangement of 4 examples Eupodiscus radiatus diatoms, the other is a 7 form test slide made by Klaus Kemp. Low magnification images (using an 20x Olympus Splan objective) of the samples on the two slides are shown below.
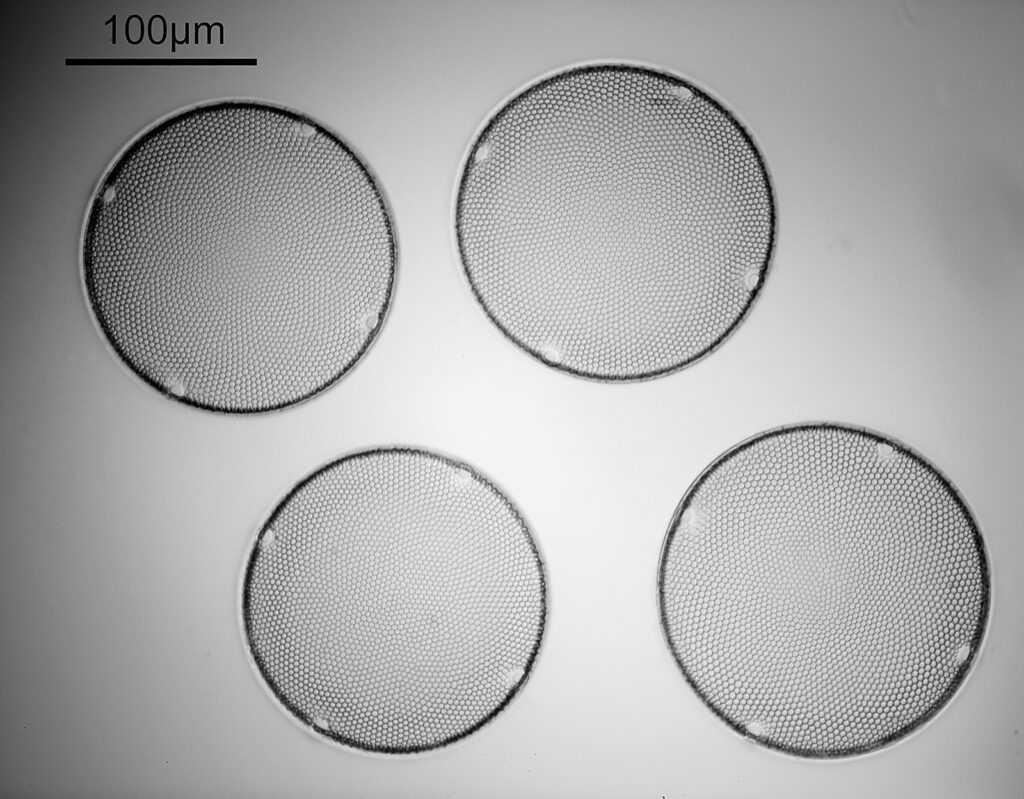

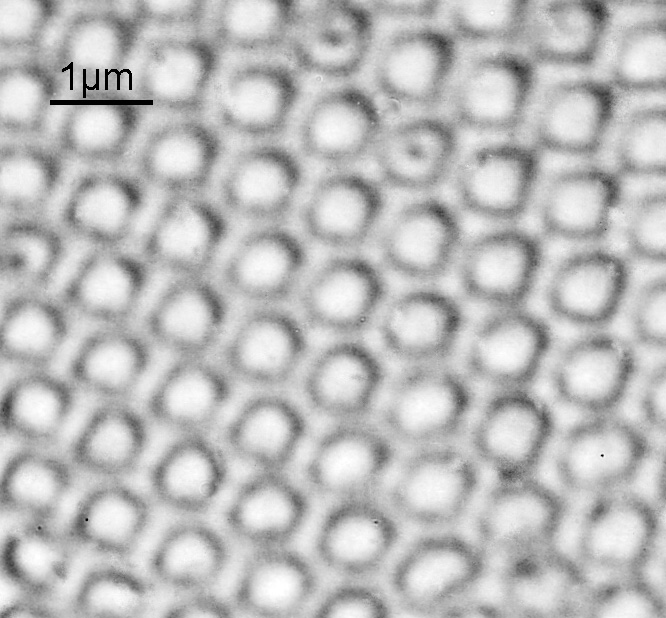
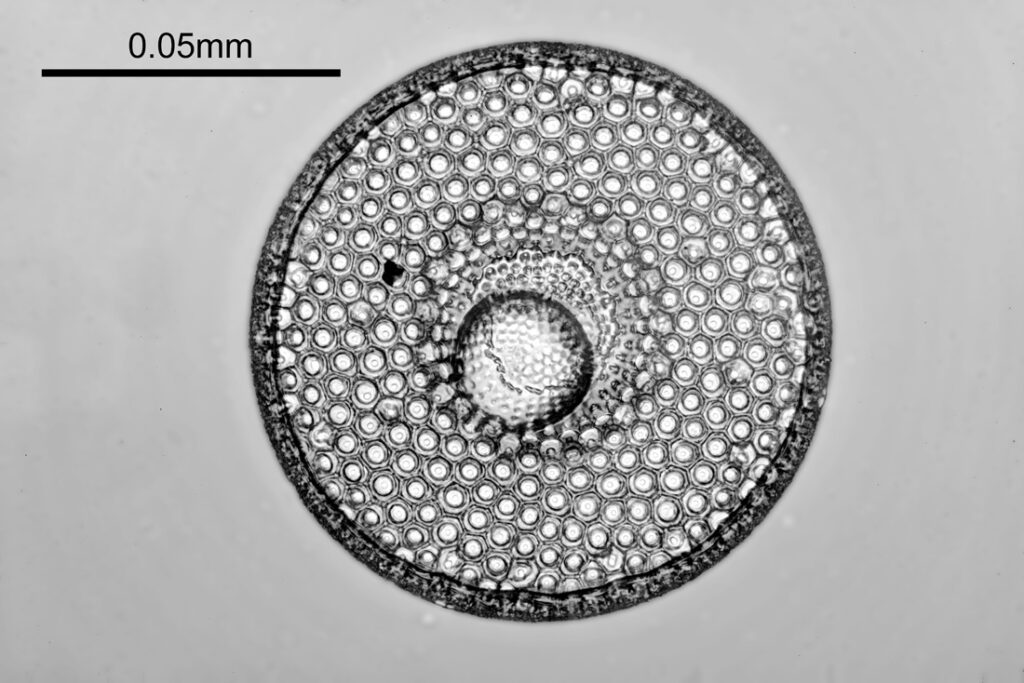
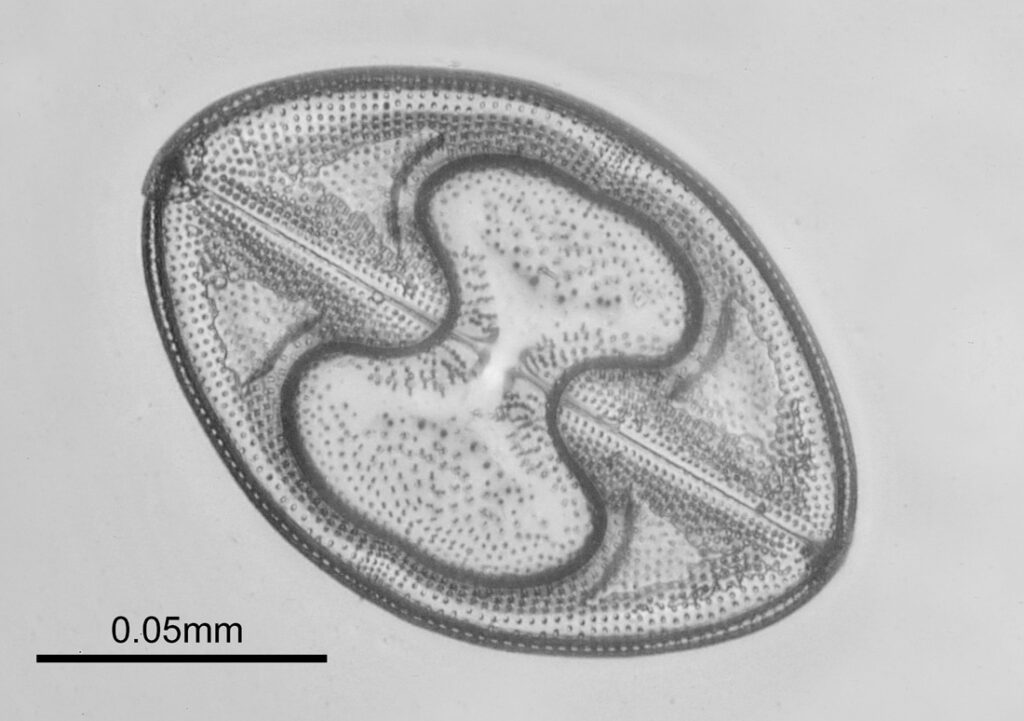
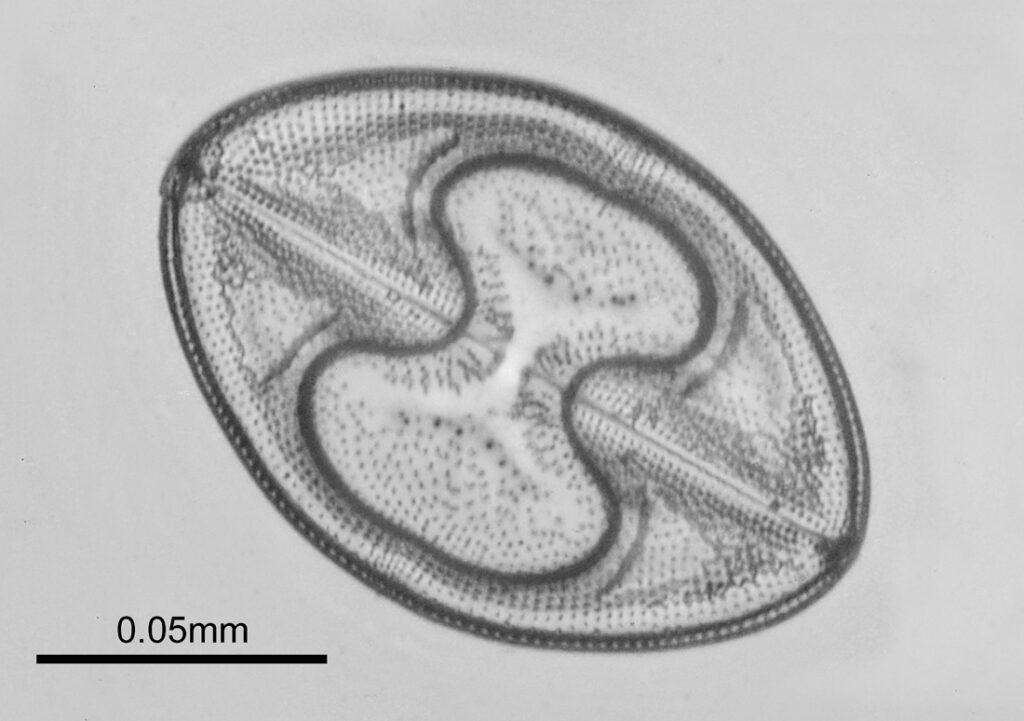
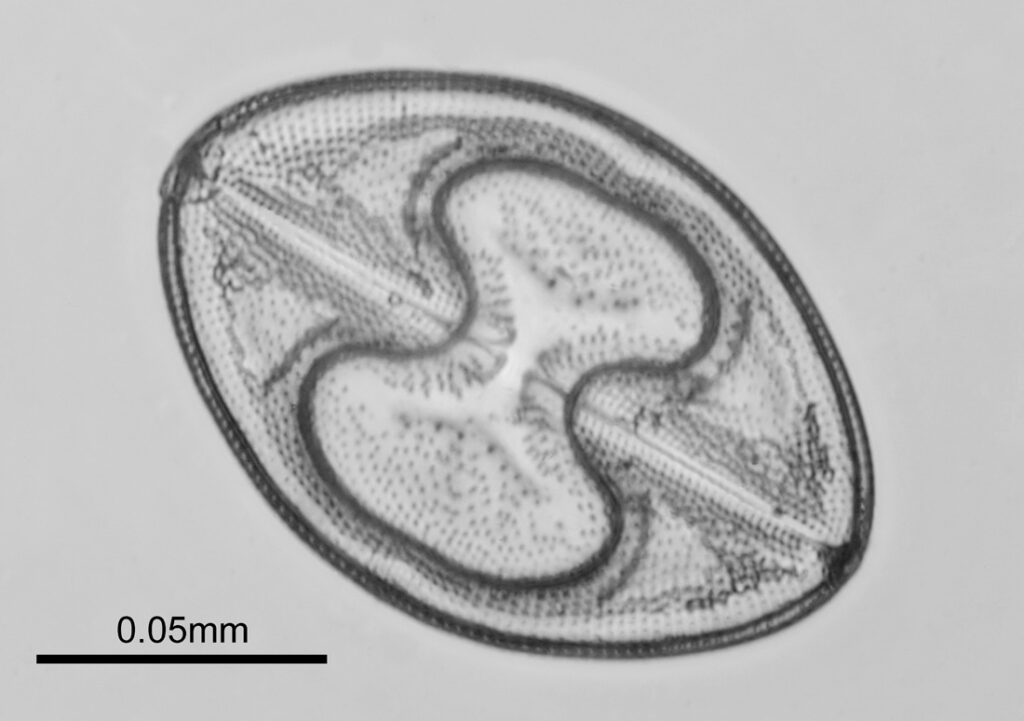
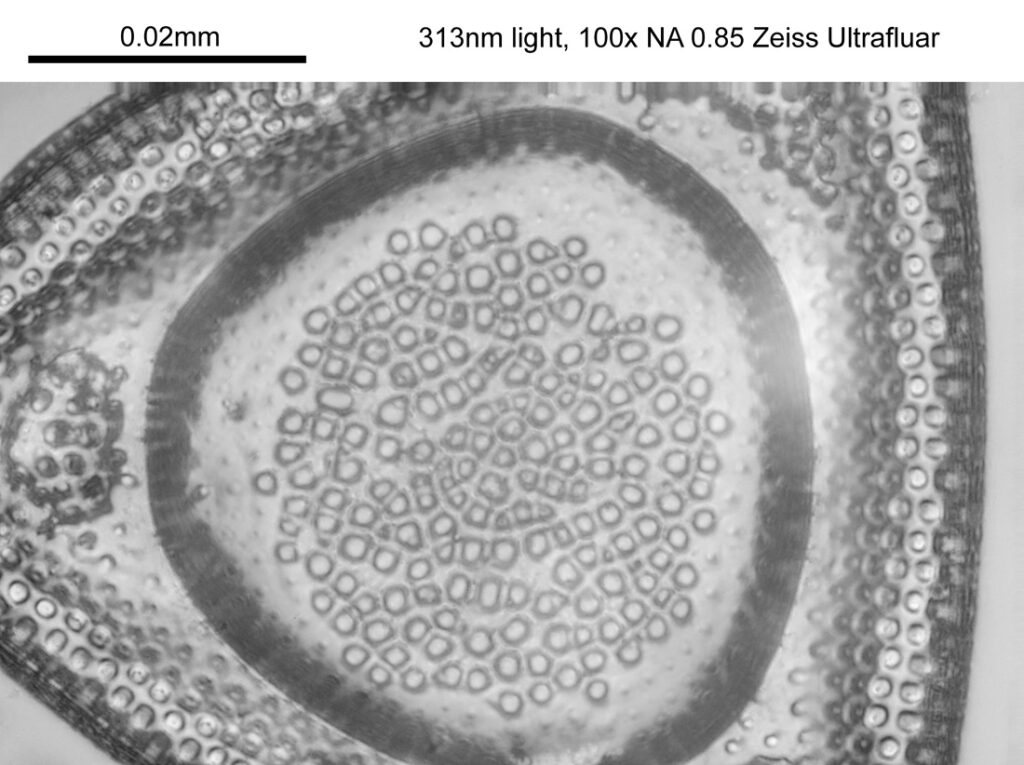
The images above where made using 405nm light on my UV microscope. How on earth anyone is able to move an arrange these things on such a small scale amazes me. But it is the structures in the diatoms that become more clearly visible at high magnification which are really fabulous. On the first slide, moving to a 60x objective (Olympus 60x Splan Apo), gives the following.
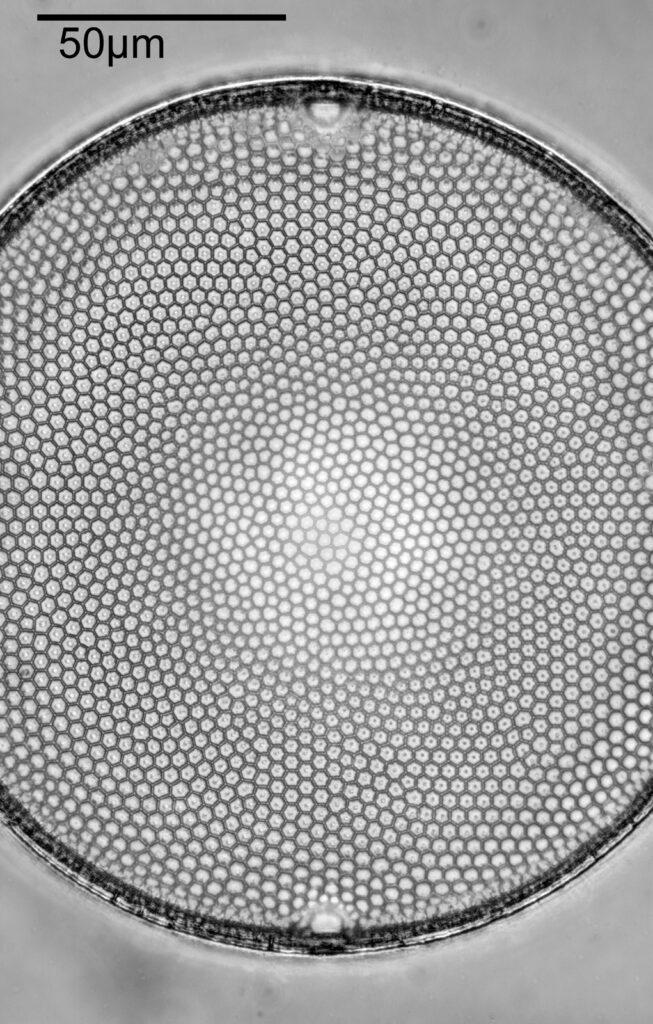
Using the 60x brings me in a little too close for this sample, but it was an objective I wanted to have a play with. Cropping the original image further, allows the structure to be more easily observed.

While the majority of the features are hexagonal, there are pentagons and heptagons spread throughout it, which allow the structure to take on the beautiful curves that it has. Not too bad for an algae.
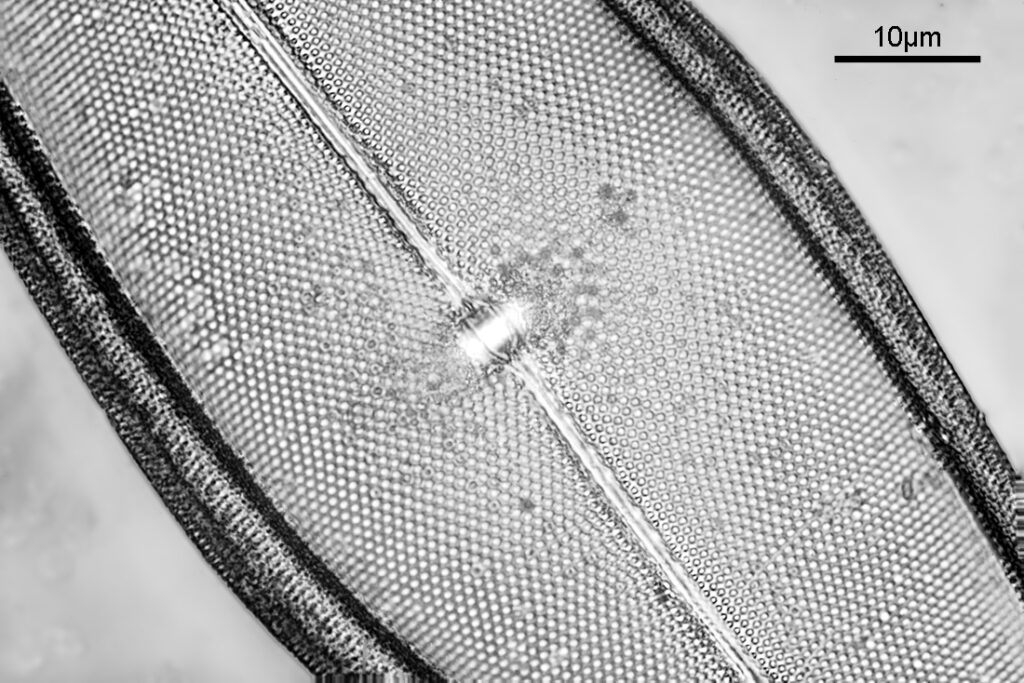
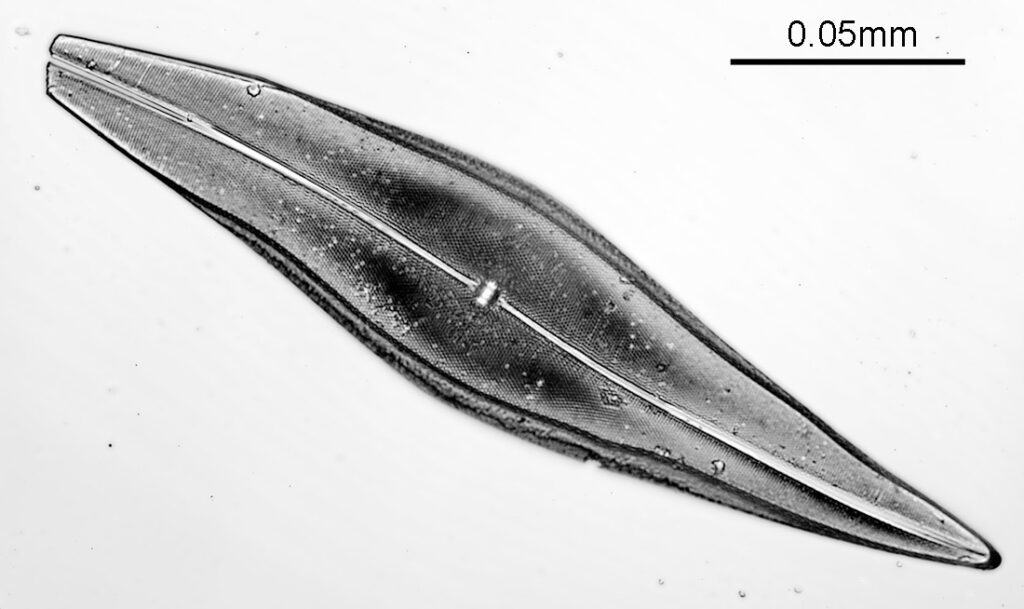
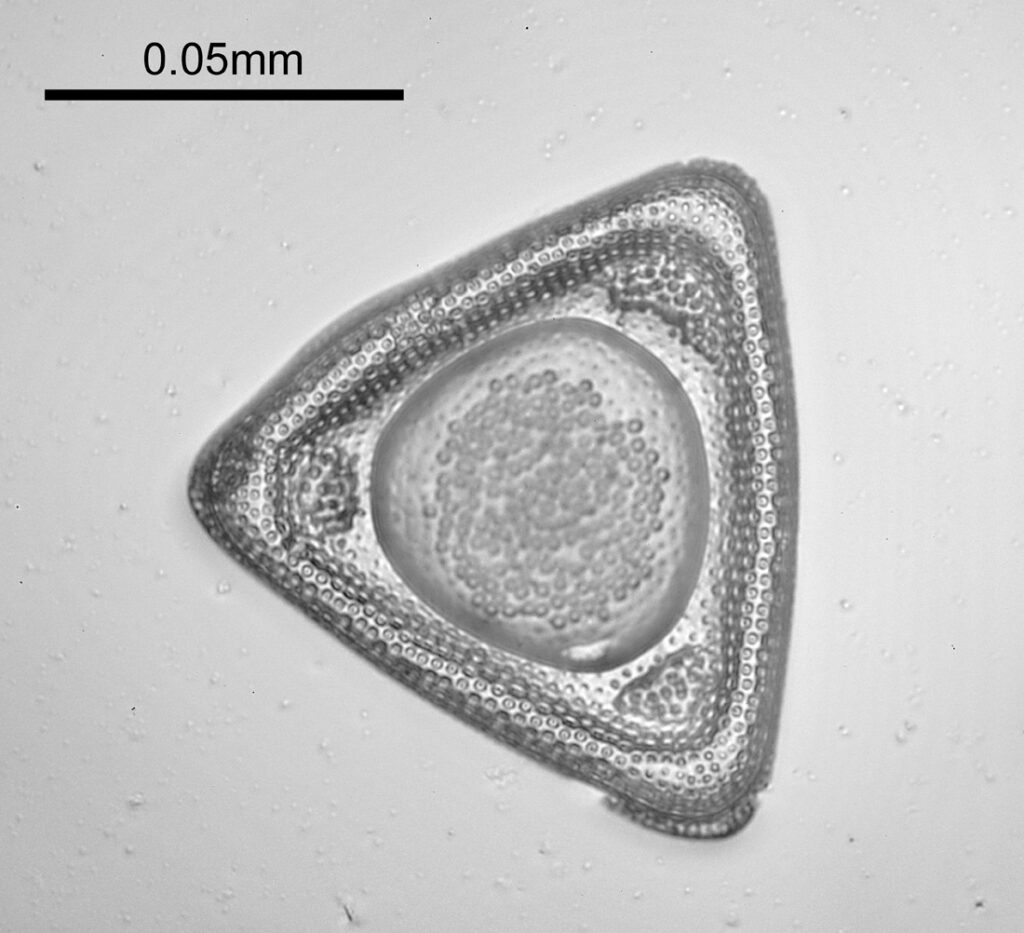
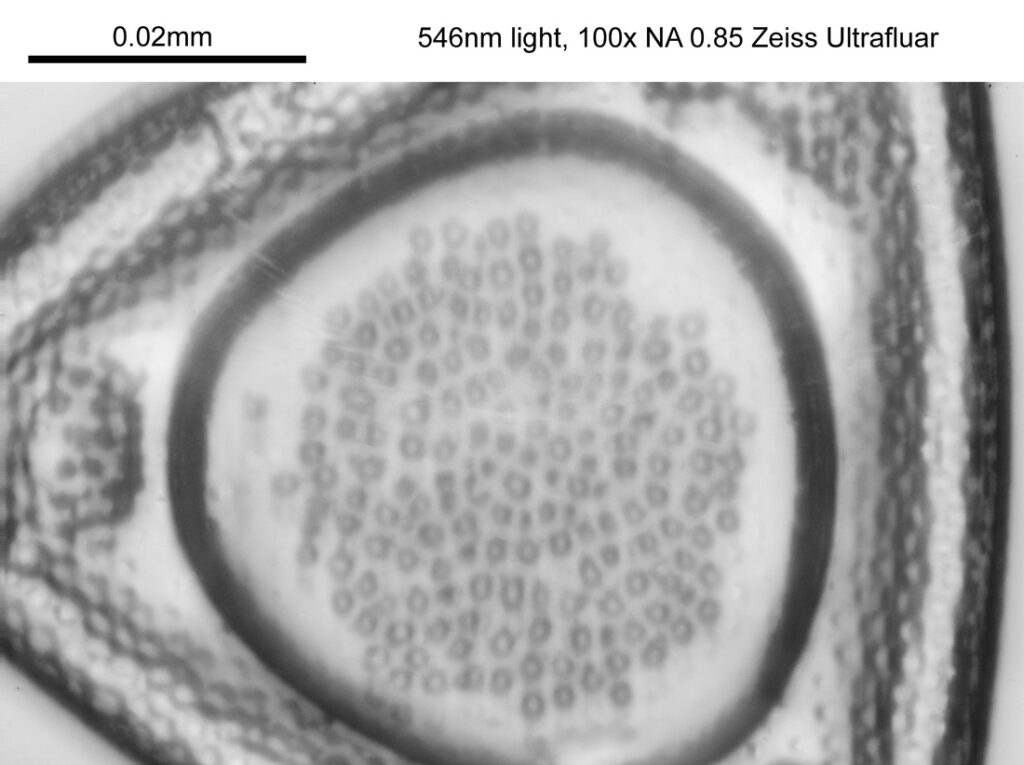
Moving into the Klaus Kemp slide, I decided to look at the one which was third from the right (Pleurosigma angulatum). This one has one nice small features and makes of a good one to look at. Here it is, again with the 60x Olympus Splan Apo objective.
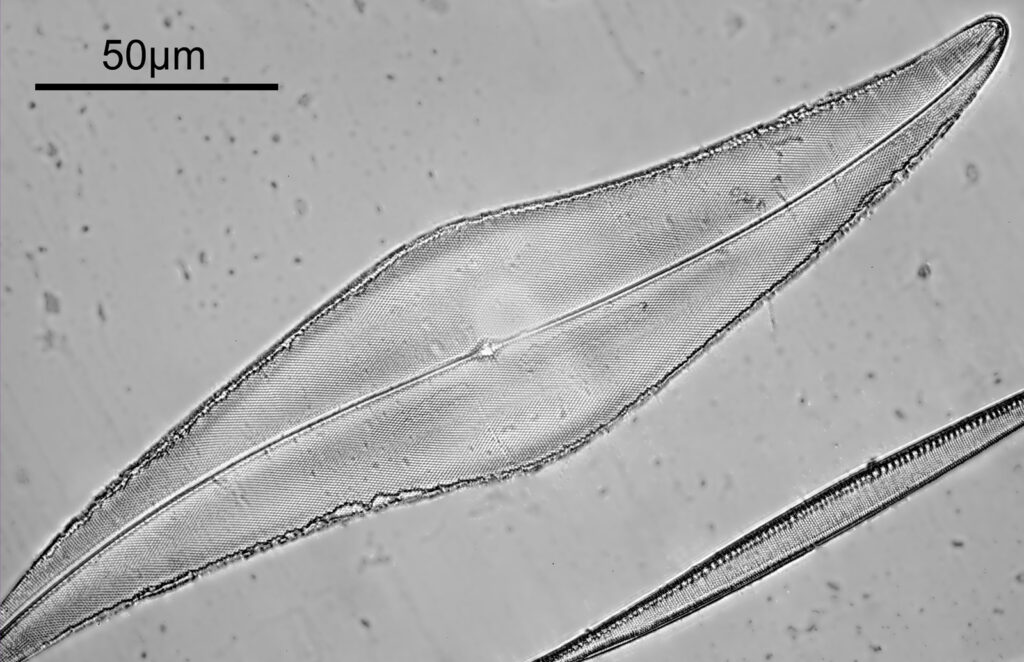
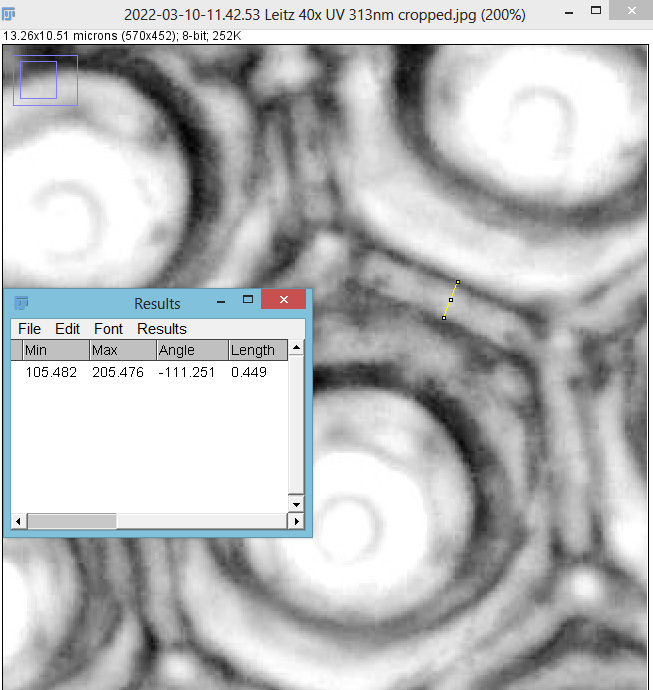
As with the Eupodiscus radiatus, the use of the 60x was slightly too big a magnification for the Pleurosigma angulatum (note to self use a smaller magnification photoeyepiece next time). However this one has some really small features, as can be seen in a cropped image below.
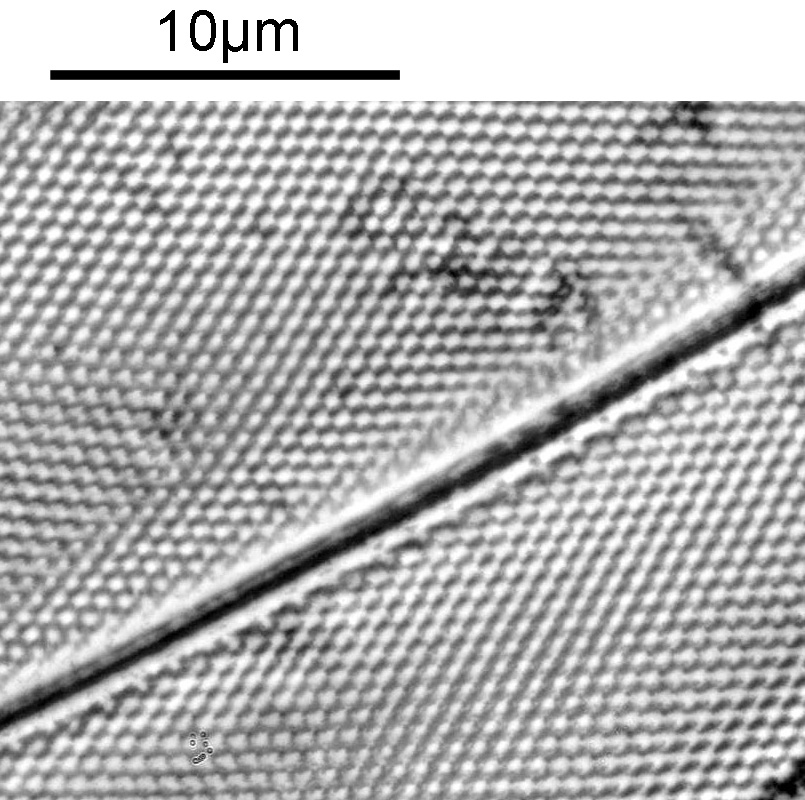
The repeating dot like features in the image above have been reported in the literature to have about 16 per 10 microns, and that is indeed what I see here.
I used 405nm light here, as this provides some improvement in potential resolution vs normal white light. Using 405nm gives a nice boost to resolution without all the hassle of going to full UV imaging, and it means being able to use normal slides, coverslips, objectives and condensers. However this time I didn’t quite get the setup right as there is a hotspot in the middle of the 60x images and a bit of fall off in one of the 20x ones. I actually cheated a bit as well and used glycerine as the immersion fluid for the 60x objective, rather than oil. This was due to laziness – its easier to clean off the glycerine than oil – and perhaps I sacrificed a bit of image quality as a result. I also didn’t oil the condenser to the underside of the slide – due to not wanting to make a mess.
Before I leave todays post, I wanted to share images of the slides themselves. First the antique John Barnett one.

The John Barnett slide is actually paper wrapped, and the diatoms are in the middle of the glass in the centre of the slide. They are of course not visible at this scale. Next we have the Klaus Kemp slide.
The Klaus Kemp slide is a simpler affair, and the diatom are inside the small ring in the middle of the slide.
The world of the small can be a beautiful place and for a relatively small outlay is within the reach of anyone with a simple microscope. I’m no expert on diatom imaging (check out the Photomacrography forum for some amazing images) but I just find it endless fascinating to look at them. Thanks for reading and if you’d like to know any more about this or my other work, I can be reached here.