Today’s post poses a question. When does the editing of images become too much? When doing microscopy, images have to be edited. Dust on the sensor needs to be removed, the background needs smoothing, noise needs reducing and images need sharpening. The question here though, is what is acceptable editing and what isn’t? A somewhat philosophical issue, but something I found myself thinking about with an image recently. Here’s the image, a Brightwellia coronata diatom from a strew slide of Totara, Oamaru, New Zealand, made by Ray Parkinson (reduced in resolution from the original 3500×3500 image for sharing here).
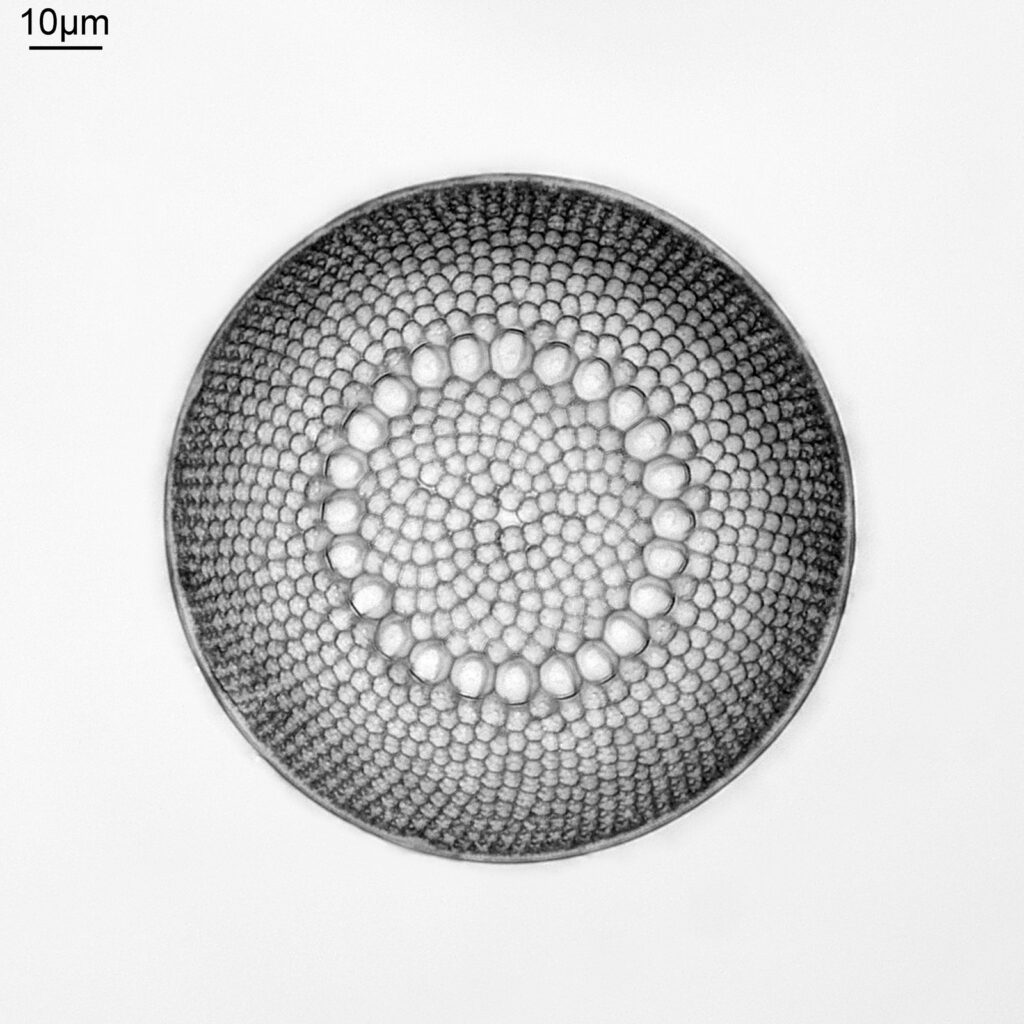
The image above was taken using my Olympus BHB microscope, using 450nm LED light, and a 63x Leitz Pl Apo NA 1.40 oil immersion objective. Brightwellia coronata is a beautiful diatom, and one which is very difficult to find undamaged. I have various strew slides from Oamaru which have broken ones on them, and some which are almost but not quite intact. A few days ago I was looking through this slide from Totara, as I’ve been having some good luck with strew slides recently. After looking through most of it, and seeing fragments I saw what looked to be an intact one, buried in the middle of a load of others. This was what I saw with a 10x Nikon Plan Apo NA 0.45 objective – the B. coronata is in the middle of the image.
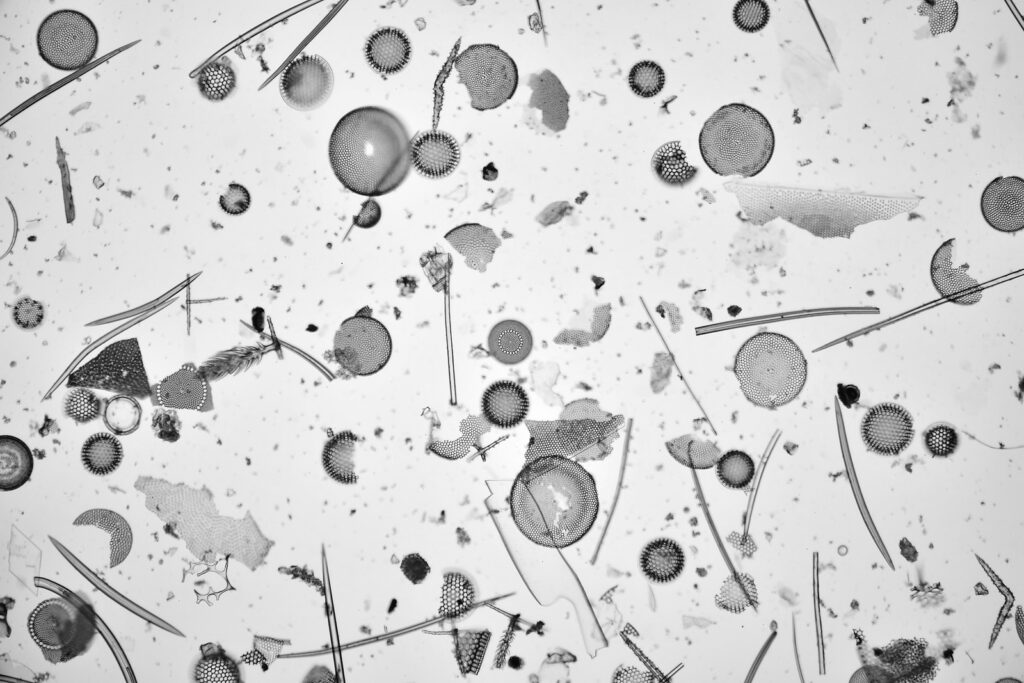
As can be seen from the image above, its a busy slide and with quite a bit of debris across it. In addition to what looks to be a small B. coronata in the middle of the image, there are a couple of larger broken ones to the left and lower left of the one in the middle. In this low magnification image the main one looked to be intact, but there was some dirt at the edge of it at the 8 o’clock position. When I did a high magnification image of it using my 63x Leitz Pl Apo NA 1.40 objective, the piece of dirt was just covering the very edge of the diatom. Removing most of the dirt which wasn’t over the diatom was simple enough using the stacking software (Zerene) and blank image. However I started wondering, can I remove that piece of dirt in Photoshop by cloning a short section of the edge of the diatom that I can see and pasting it over the bit where the dirt was? Below are three crops of the area in question from the stacked image to show what I mean.
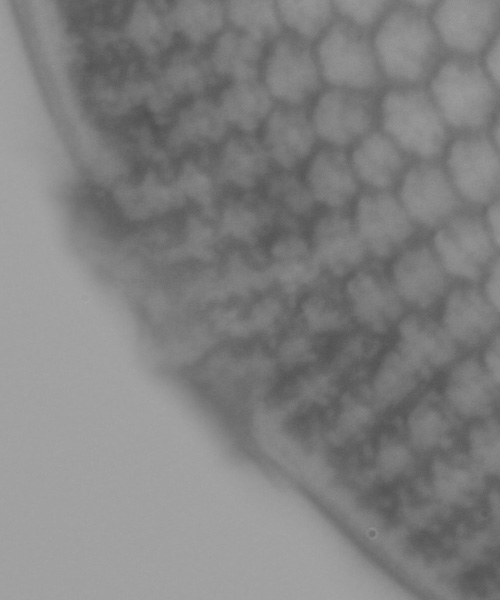
First is the stacked image from Zerene, the bulk of the dirt which is not on the edge of the diatom has been removed. It obscures the very edge of the diatom’s rim over roughly 3% of the overall circumference.
The diatom underneath did not look to be broken, it just looks like the piece of dirt is laid on top of it. The next image is with a section from the rest of the visible edge of the diatom cloned and pasted over this bit with the dirt and smoothed out (and I’ve removed some dust on the sensor – the circle at the lower right of the image above).
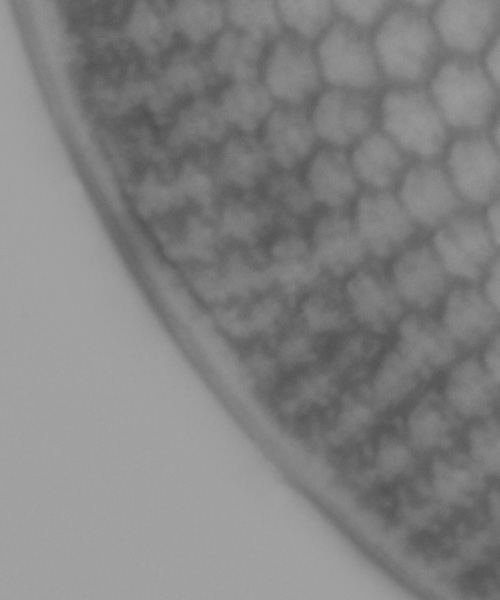
I then went through the final editing (removal of dirt from the sensor, denoising, sharpening, smoothing, contrast etc), and ended up with the image shown at the beginning of this article. The crop of the area of interest after all this is shown below.
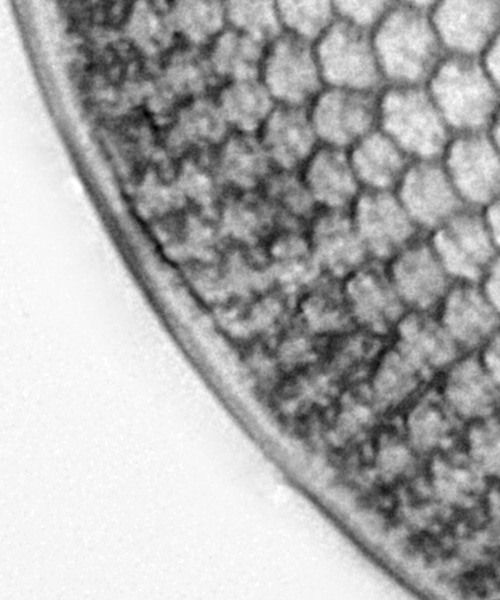
And this is where I come back to my original question? When does editing of the image become too much? As a matter of work flow, I play around with brightness, contrast, sharpness etc all the time. I clone out areas where there is dust on the sensor, making a best judgement as to what to replace it with by looking at the surroundings. I remove dirt and other diatom fragments from the background of the image, and smooth it out so it doesn’t detract from the main subject. However what I don’t do with a broken diatom is replace parts of it to make it look whole again. At the end of the day I am trying to image what is there. This case is a bit of a middle ground. The diatom did not look broken from what I could see, and the dirt was covering a small part of the edge of it. I made a judgement call as to what the bit that was covered would look like and replaced it. It certainly makes the image look nicer, but is it still relevant as an image? This is one of those images where I could argue either way. If I were to put this image in an article I would feel the need to disclose that it has been edited (and how it has been edited), but this is just how I work.
Before I wrap up, here’s the slide.

Image processing is a necessary part of dealing with microscope photos. What we deem as acceptable comes down to a number of factors including our own personal preferences and where the final image will end up being used. I hope you found this interesting, and if you’d like to know more about my work I can be reached here.
