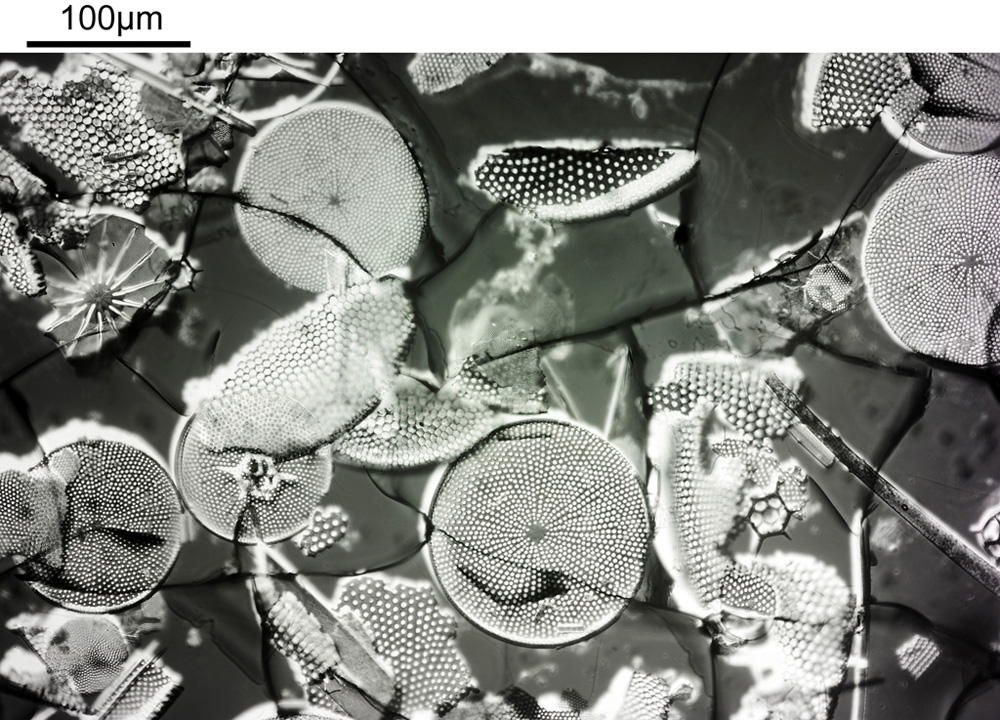
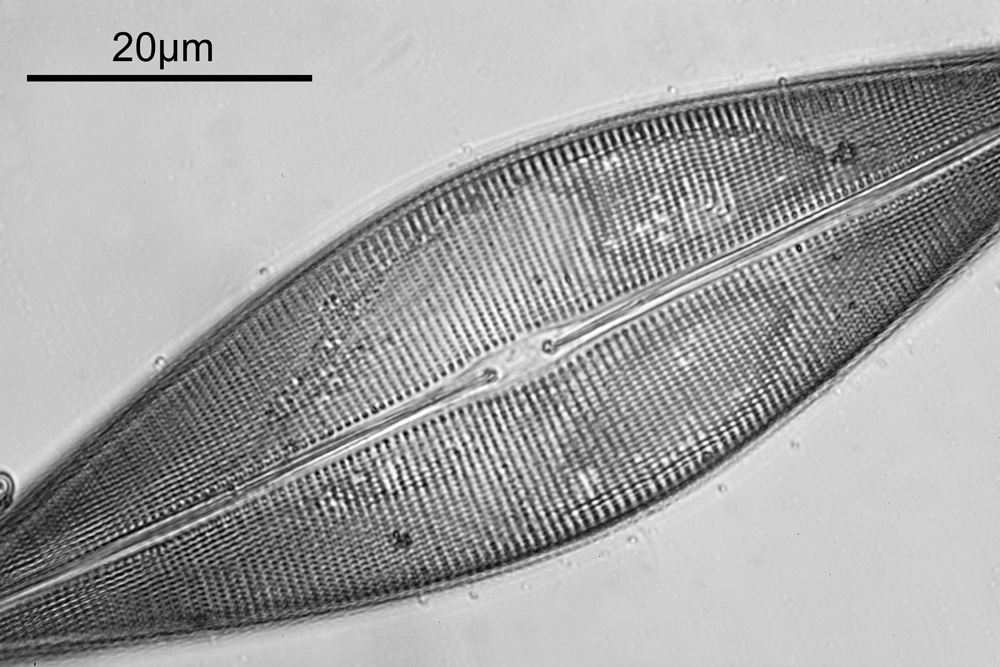
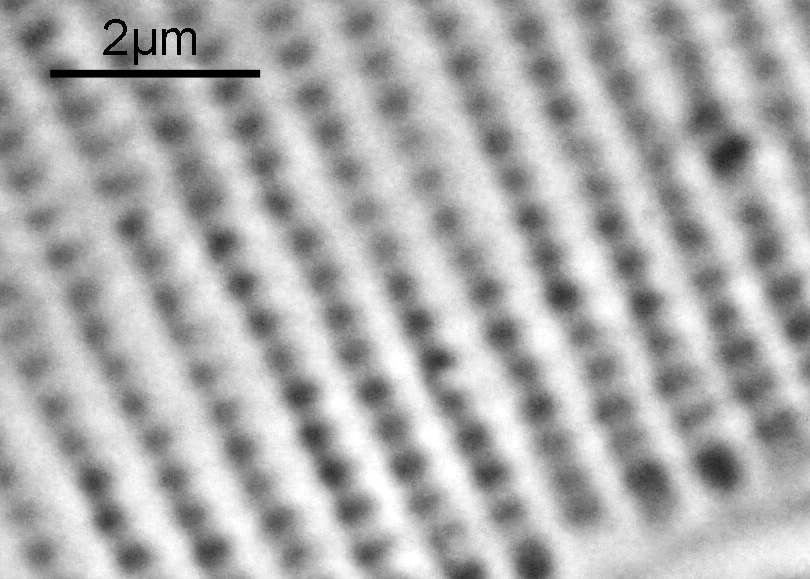
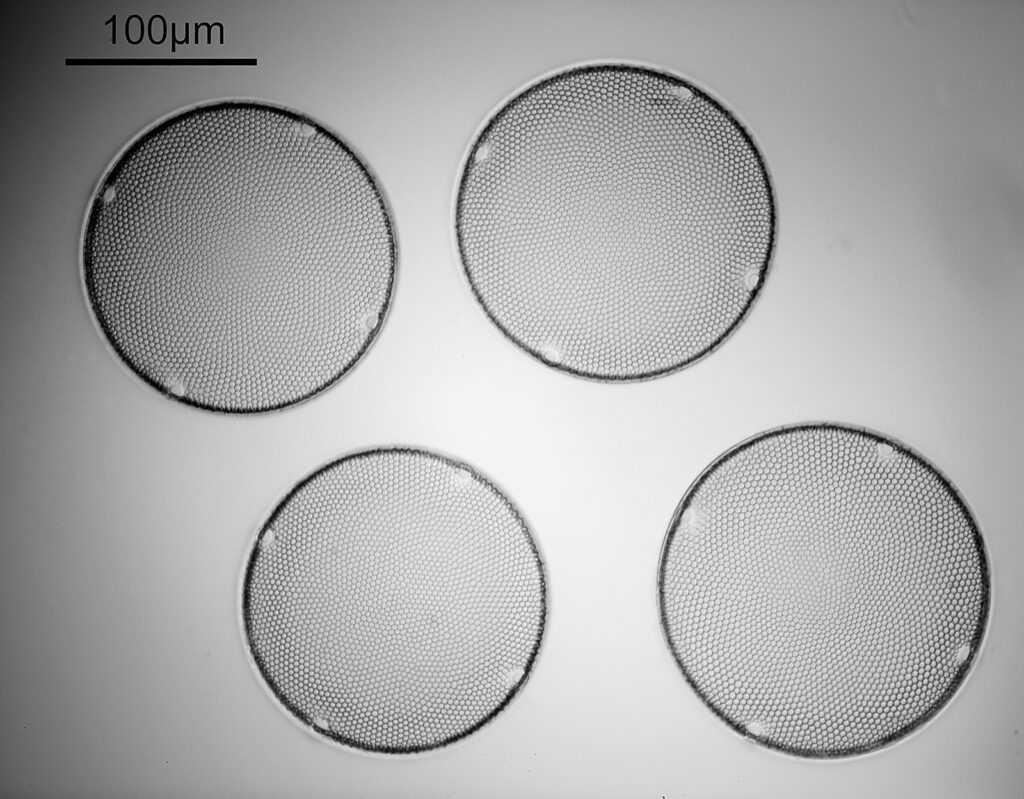
Ok, perhaps not the catchiest title for today’s post. However, it’s an unusual post. When you were young, like me, I presume there were things you were told not to do – “don’t grab stuff out of a hot oven without gloves”, “don’t charge up the cat with static electricity by stroking it for 5 minutes”, etc, etc. Of course, I often did things anyway, although to be fair I never did grab stuff from a hot oven. The objective used was most definitely not designed to be used in this way and would have fallen into the ‘don’t try this’ bucket. This builds on the imaging of the Synedra superba diatom slide I share recently (see here).
So, what is this odd objective? It’s a 170x Leitz Q NA 0.5 reflecting objective designed for use with 0.18mm thick coverslips and a 400mm tube length microscope. It’s rare and unusual, and there’s a little bit about it here in one of my previous posts. I’ll show a picture of it at the end of the post, but in summary this was designed for use between 220nm and 700nm and with its own specific reflecting condenser. It wasn’t designed to be used on a 160mm tube length microscope, or with an Olympus Aplanat Achromat condenser. Hence it was done just to see what type of image it would give. Imaging was done with 405nm light and using my monochrome converted Nikon d800.
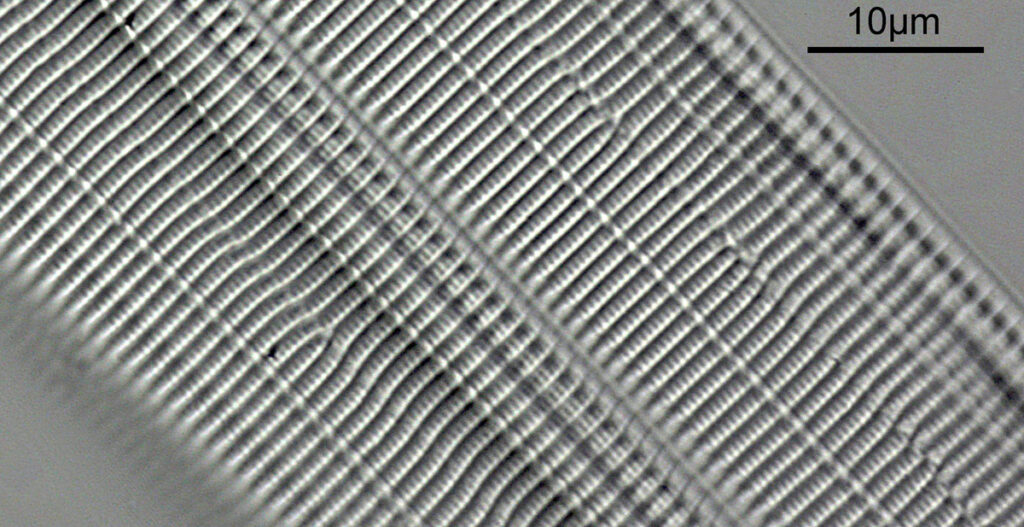
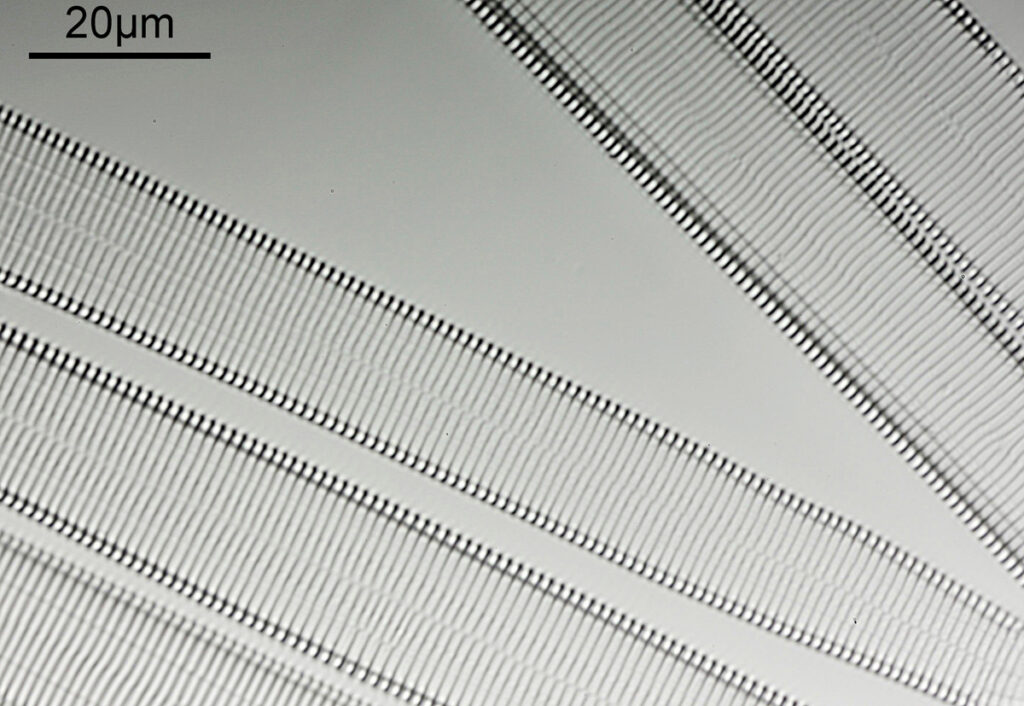
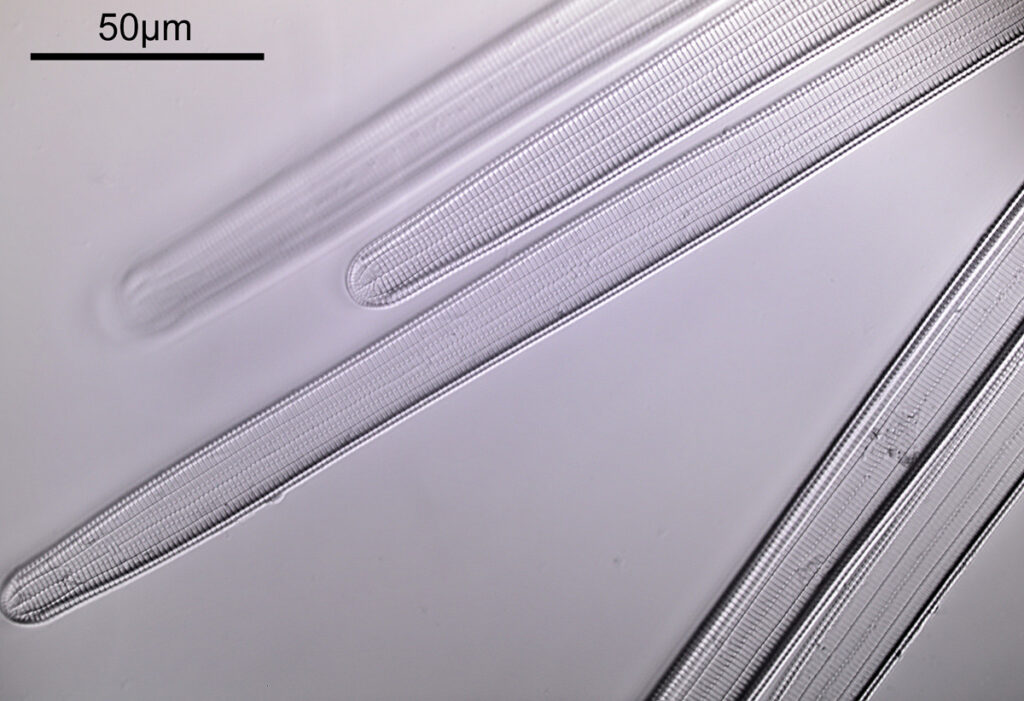
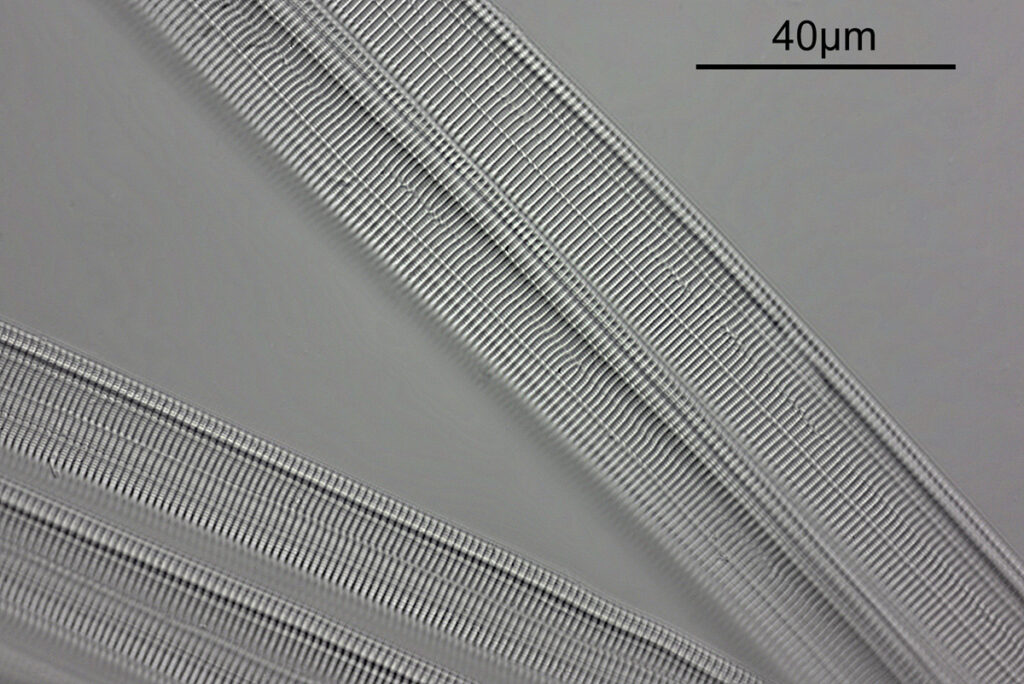
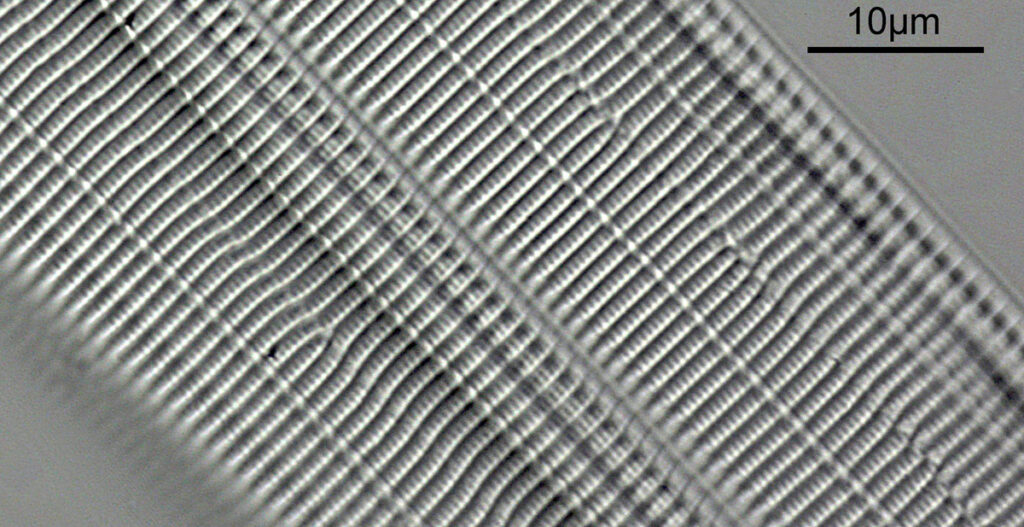
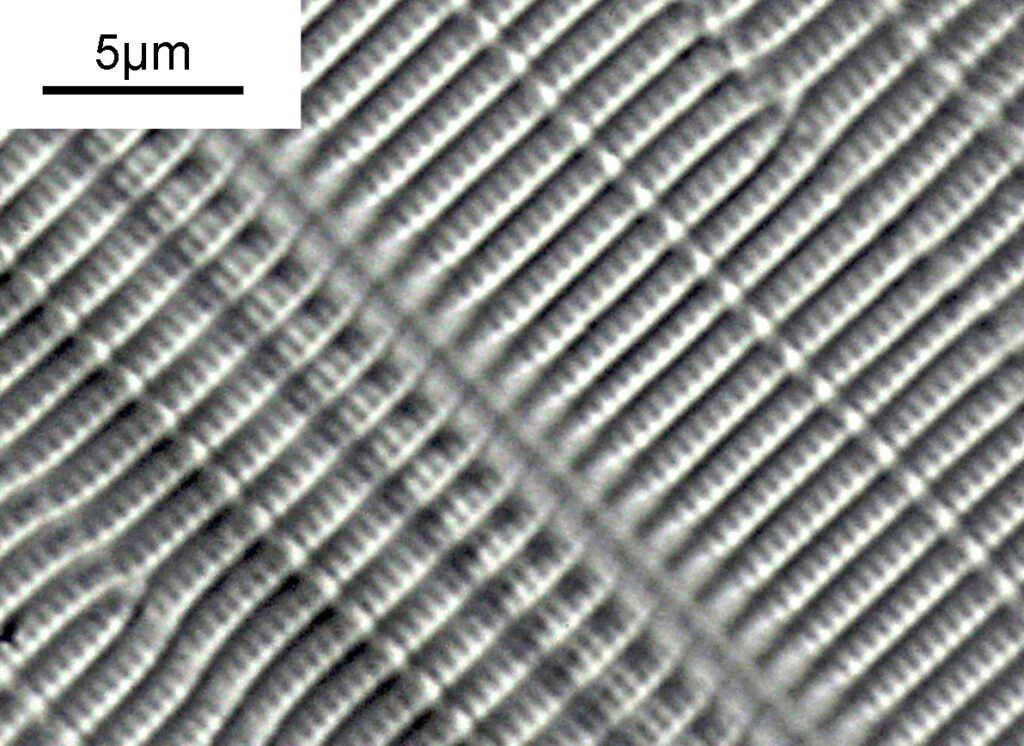
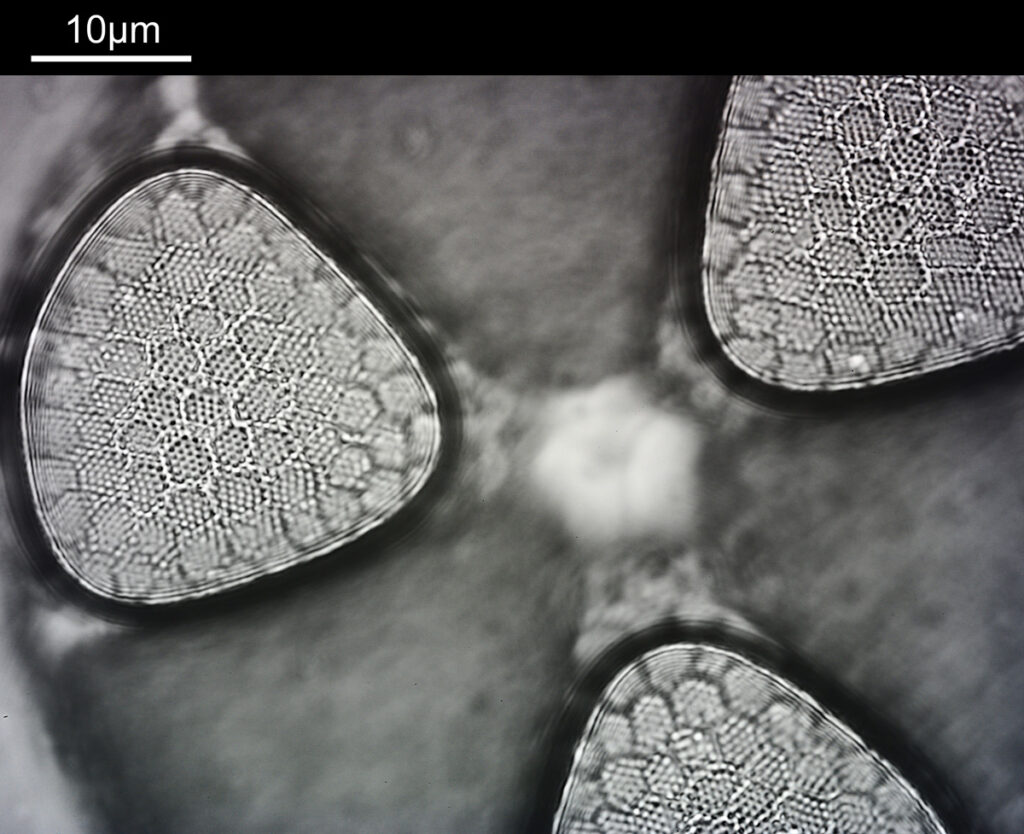
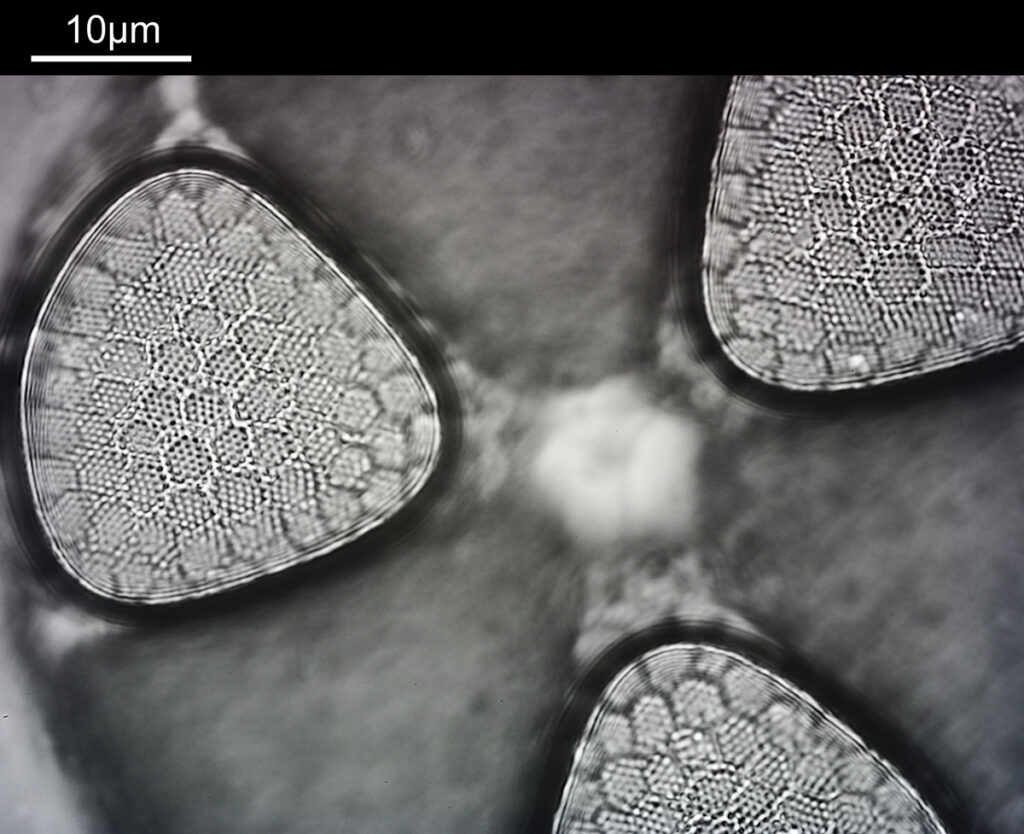
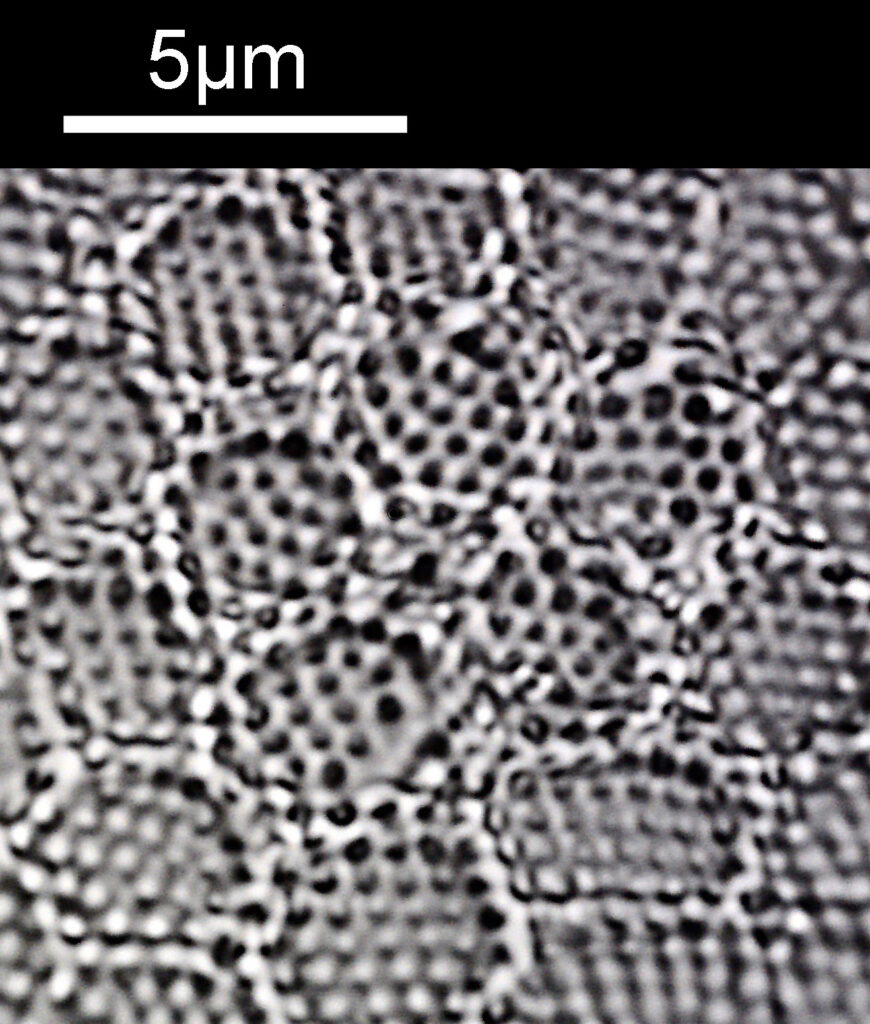
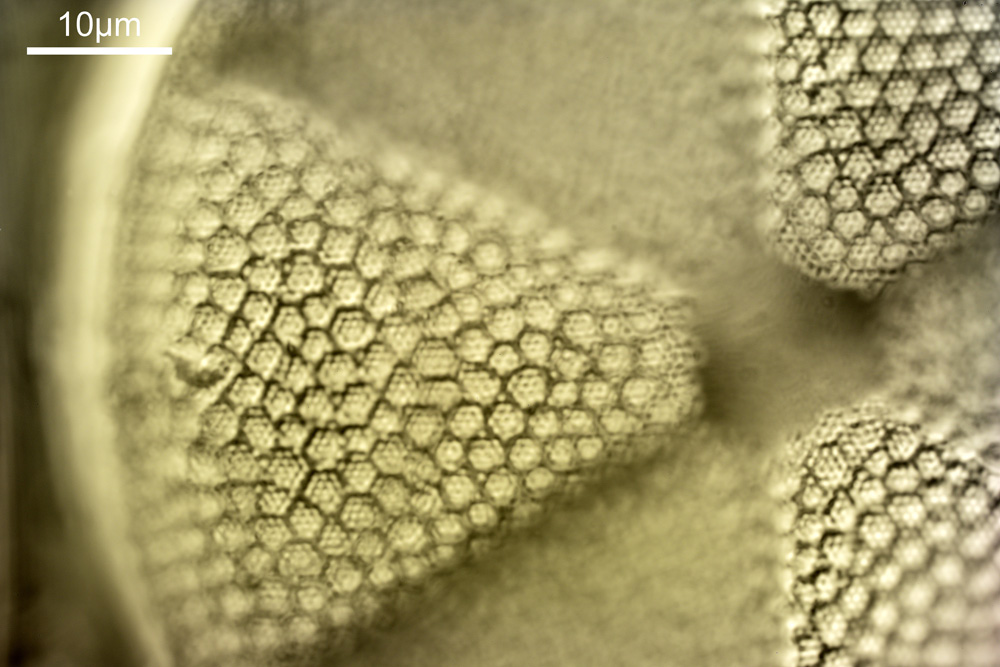
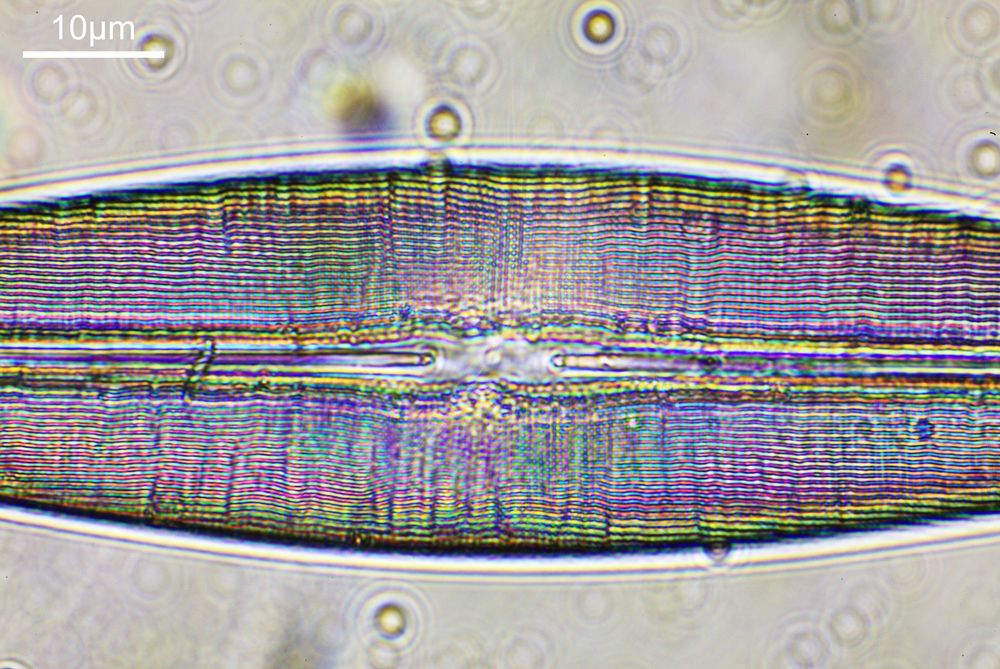
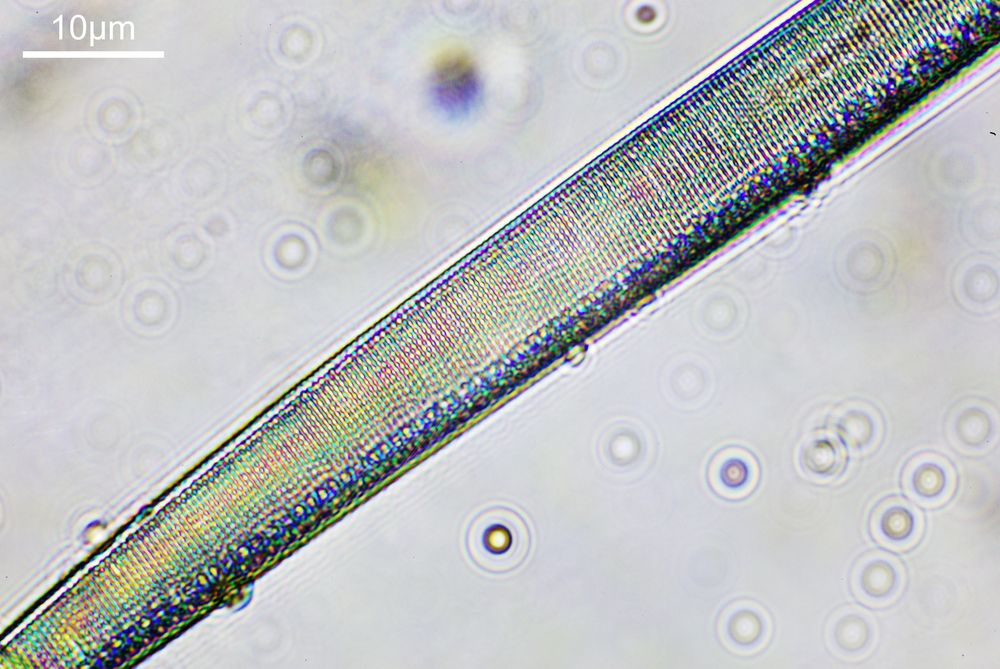
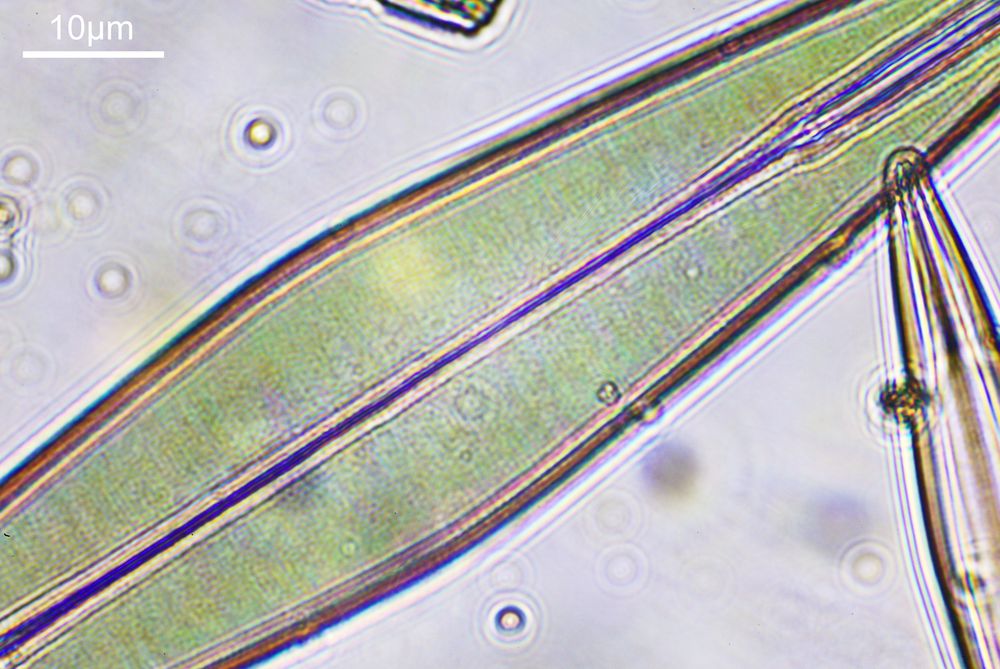
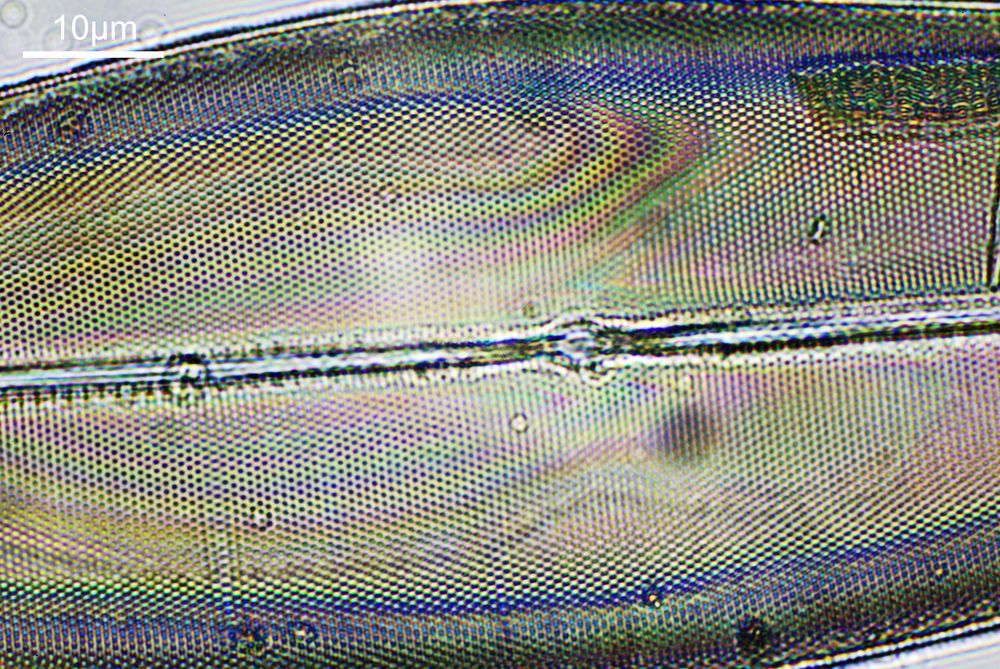
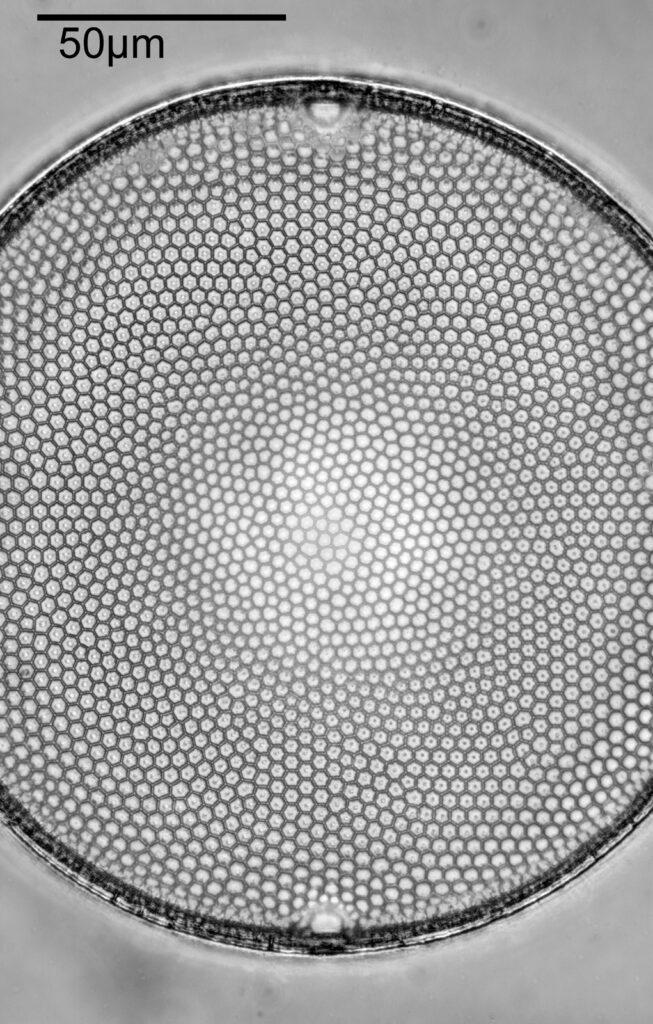
What do the images from it look like? I first tried closing the condenser right down, and was a bit surprised to see it looked like a ‘dark field’ type of image, with a black background and the diatom showing bright white.
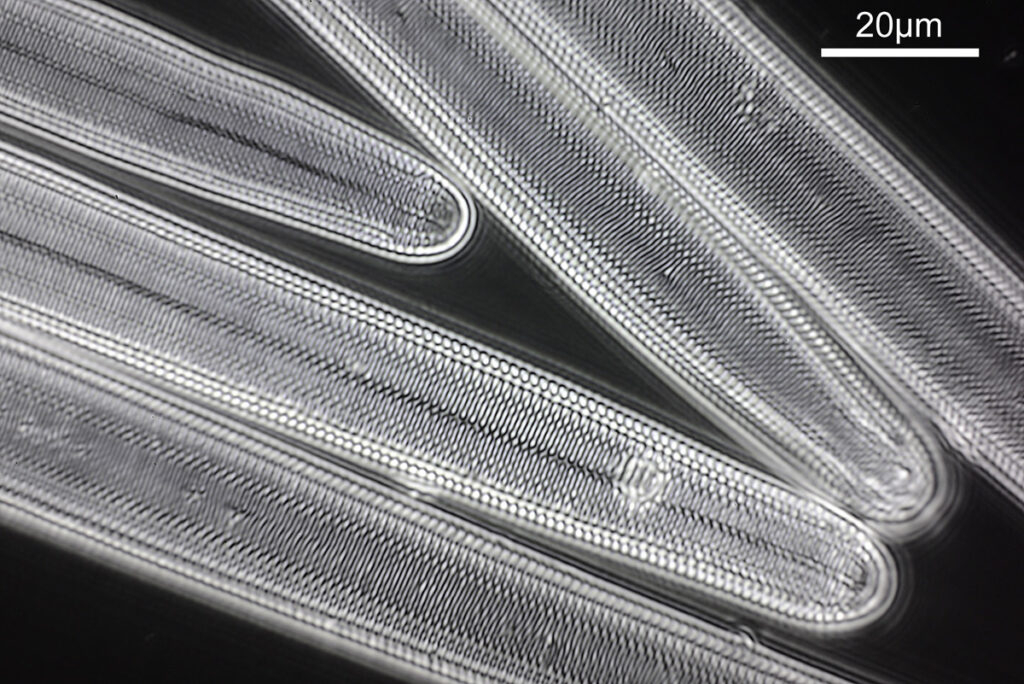
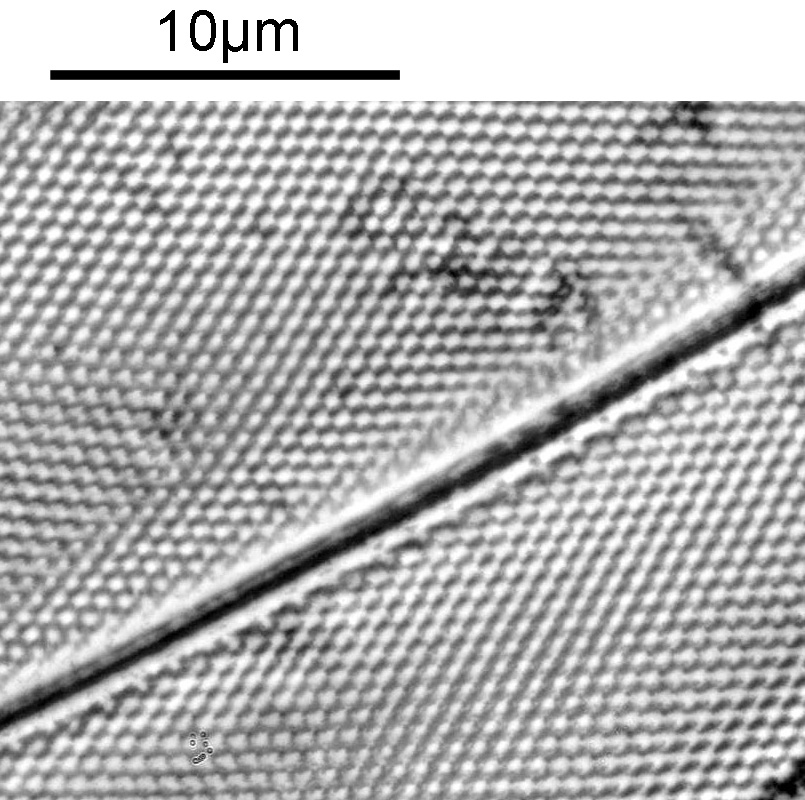
There was a surprising amount of detail here, given that this is being used way outside of the way in which it should be. Why does it look like a dark field image? My guess here is that when the condenser iris is closed right down the spot illuminating the sample becomes very small. There is a mirror in the middle of the objective (it is a reflecting design) and I guess this blocks the small spot of direct light so the only light that can pass through the objective is the indirect light scattered within the sample. However that is a hypothesis, and if anyone has any other ideas, feel free to drop me an email.
I also captured a video of the sample in this setup, where I changed the focus from above the sample to below.
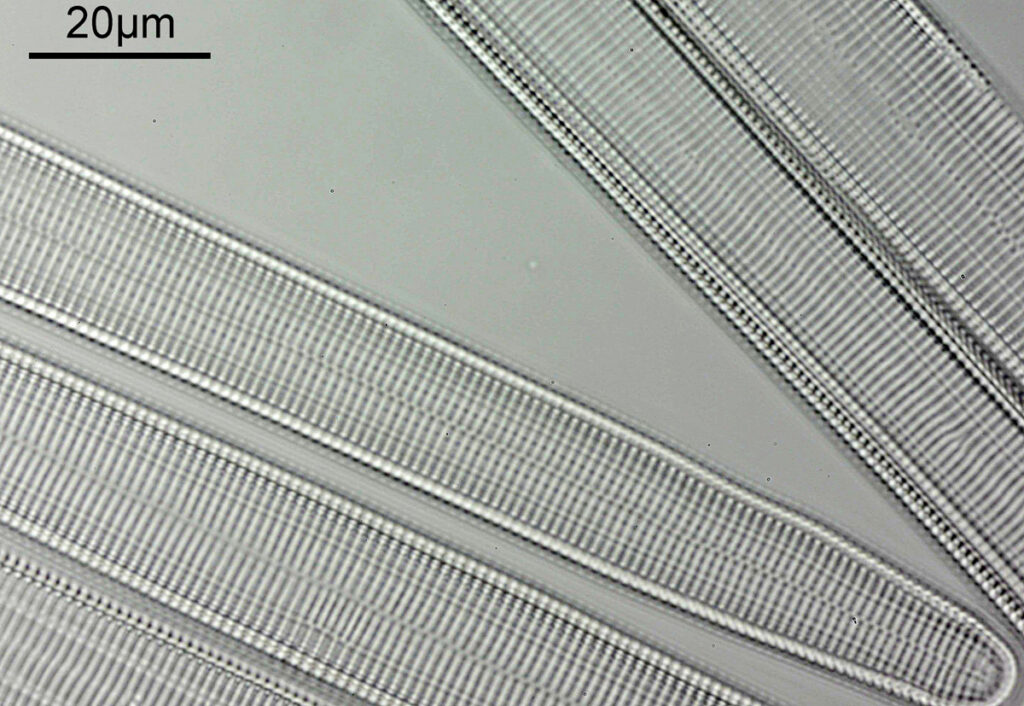
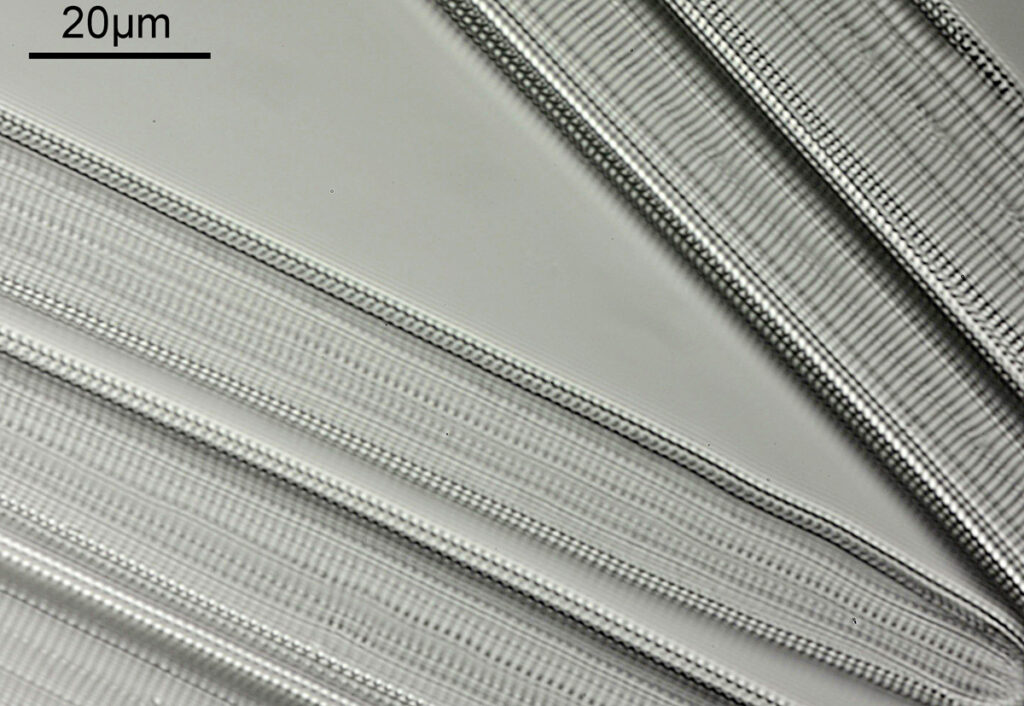
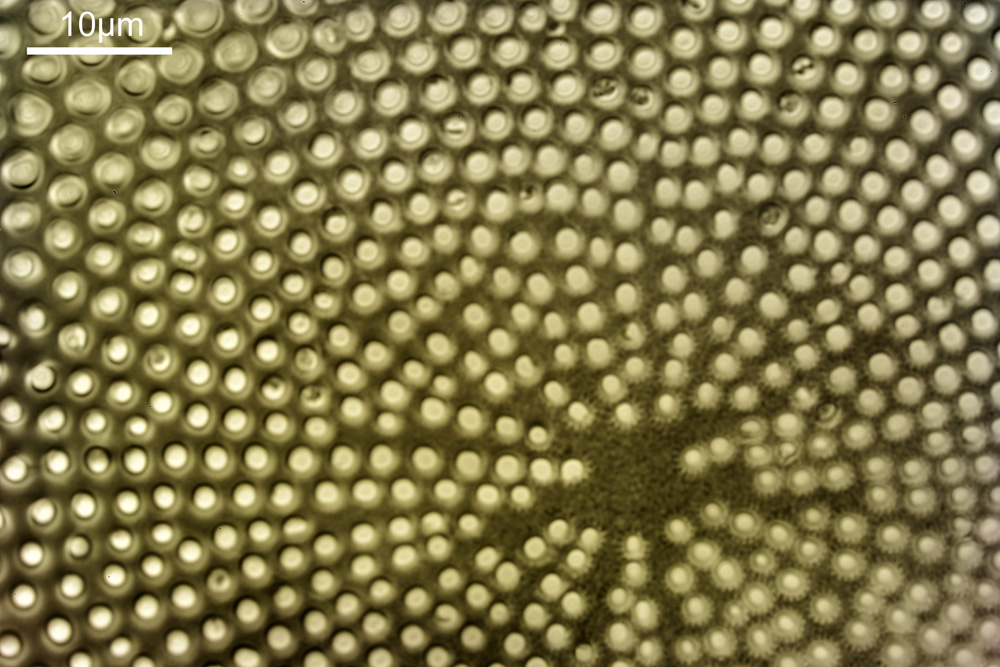
When the iris on the condenser was opened up a bit the image returned to a typical bright field appearance, but with quite low contrast.
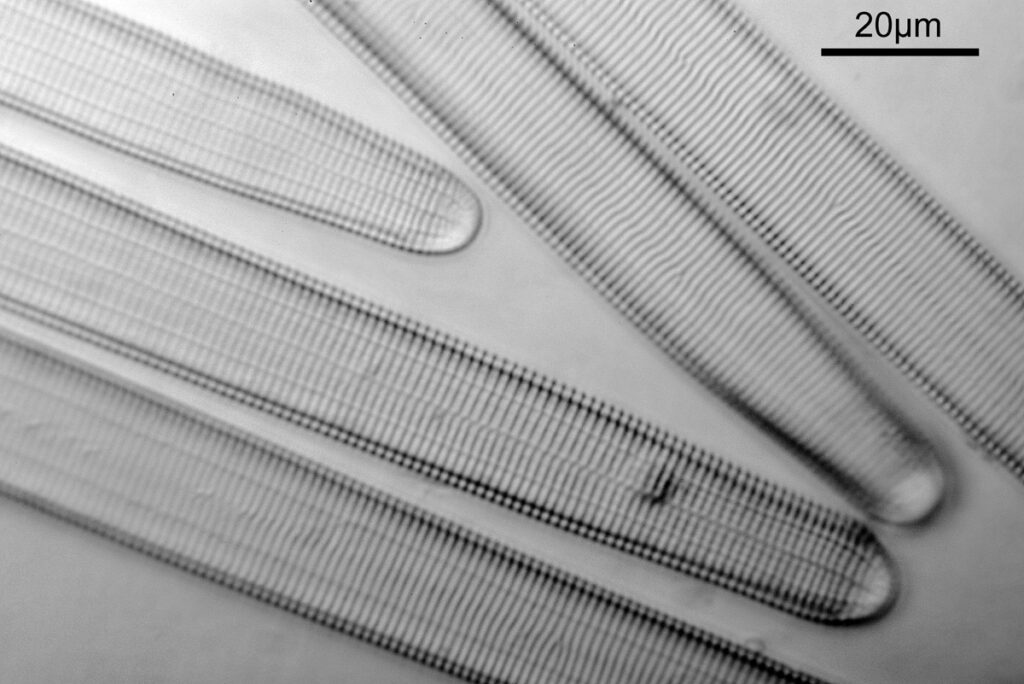
I wouldn’t want to use this objective like this, as there are plenty of ways to get better images, but with the iris closed down, the ‘dark field’ image looks quite cool, so I may try it for other samples in the future.
The 170x Leitz objective itself is shown below.


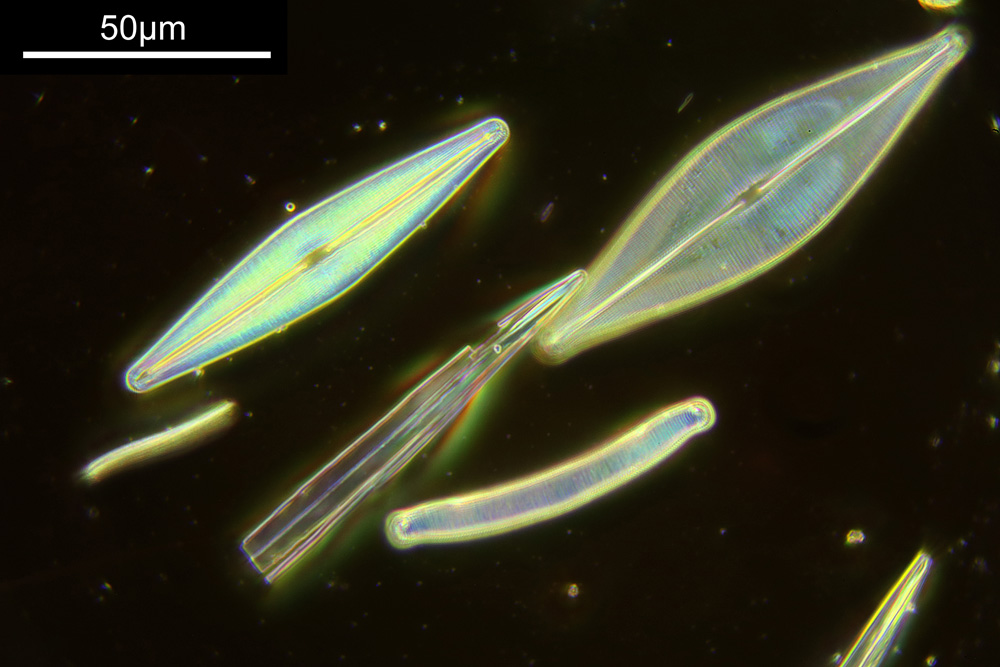
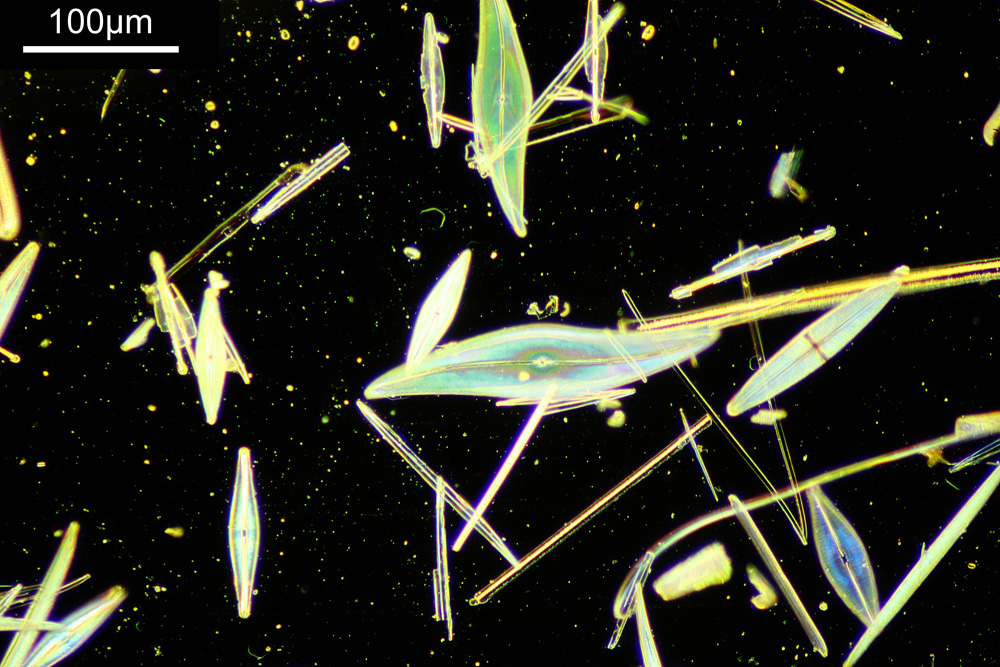
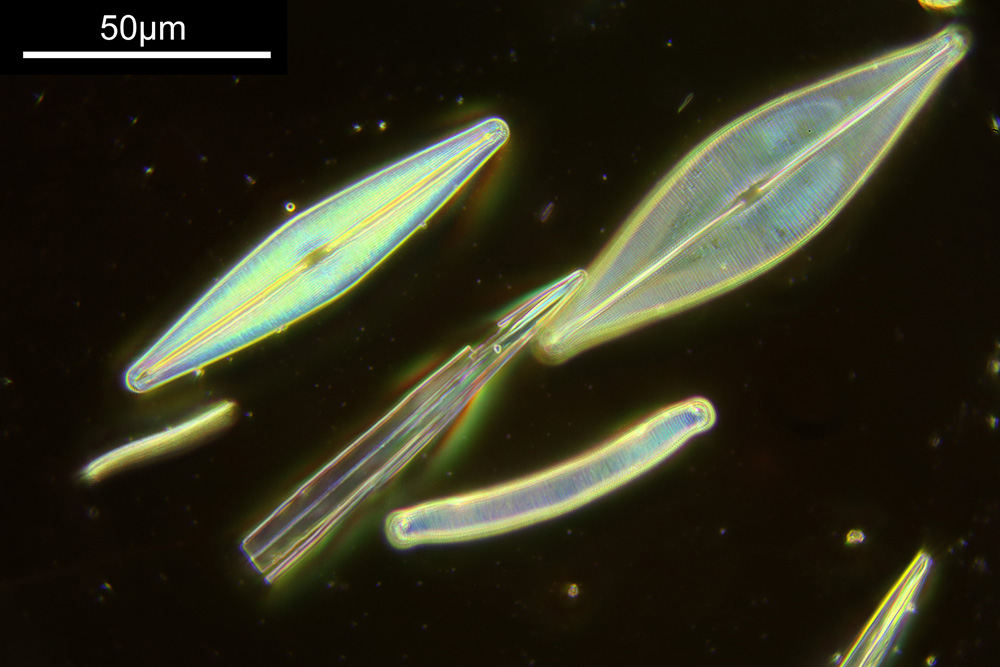
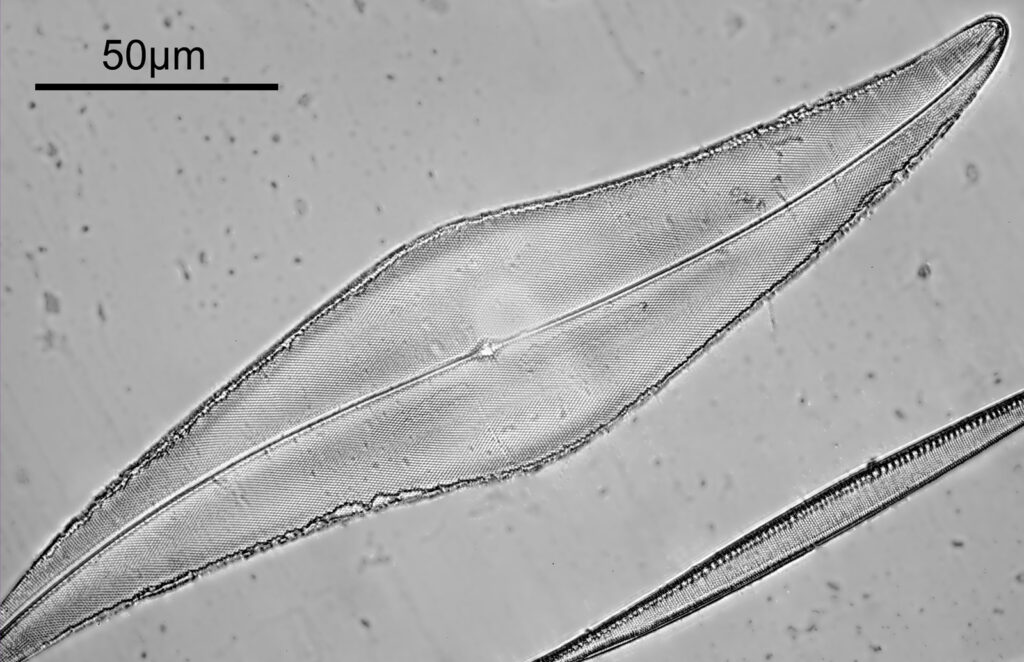
You may well have noticed something odd here. This objective is 170x, but the field of view of the images looks not too dissimilar to those from the 60x objective in my previous post on this slide. Don’t forget that this objective was designed for use with a 400mm tube length microscope, but this one is 160mm. Effectively this reduces the magnification by 2.5x times (400/160), making the magnification just 68x. This is why the images look to be similar magnification to the ones with the 60x objective. This objective does have a big brother – a 300x one – and I may try that as well in the future given the results with this one.
Part of science is doing experiments just to see what will happen, even when you think it may not work. If we knew everything that was going to happen, why do research? With this, I tried imaging using an objective which common sense said wouldn’t work, but I think it’d be interesting to try. It produced an interesting result and I learned something new. Winner. As always, thanks for reading, and if you’d like to know more about this or any other aspect of my work, I can be reached here.