A bit of a change from my normal microscopy work today. For one thing it’s colour images. Another is the subject – a thin section of coal. Also the technique used – cross polarized microscopy. Let’s get in to it.
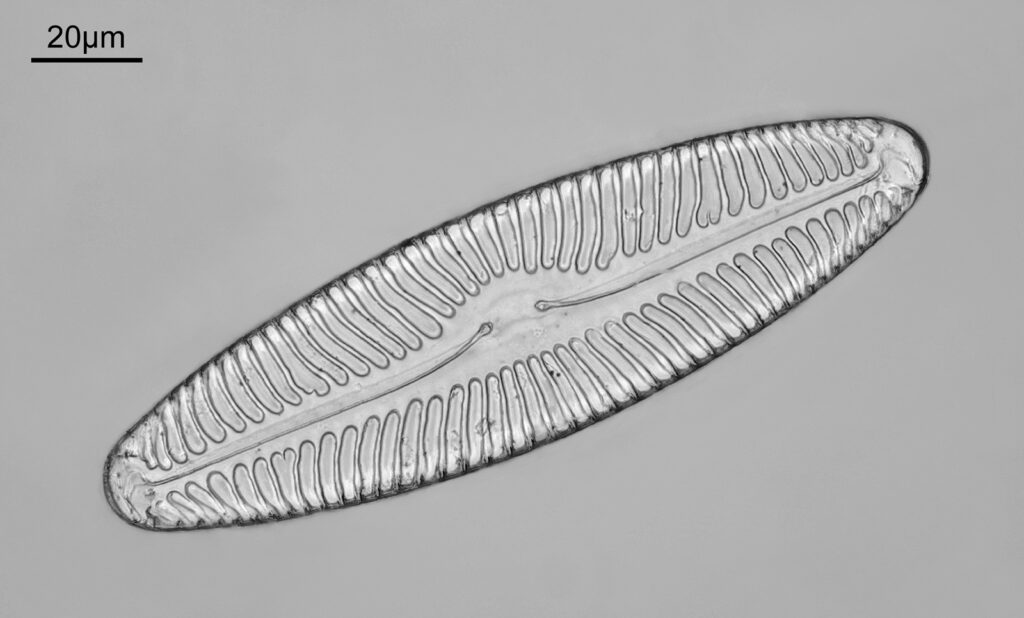
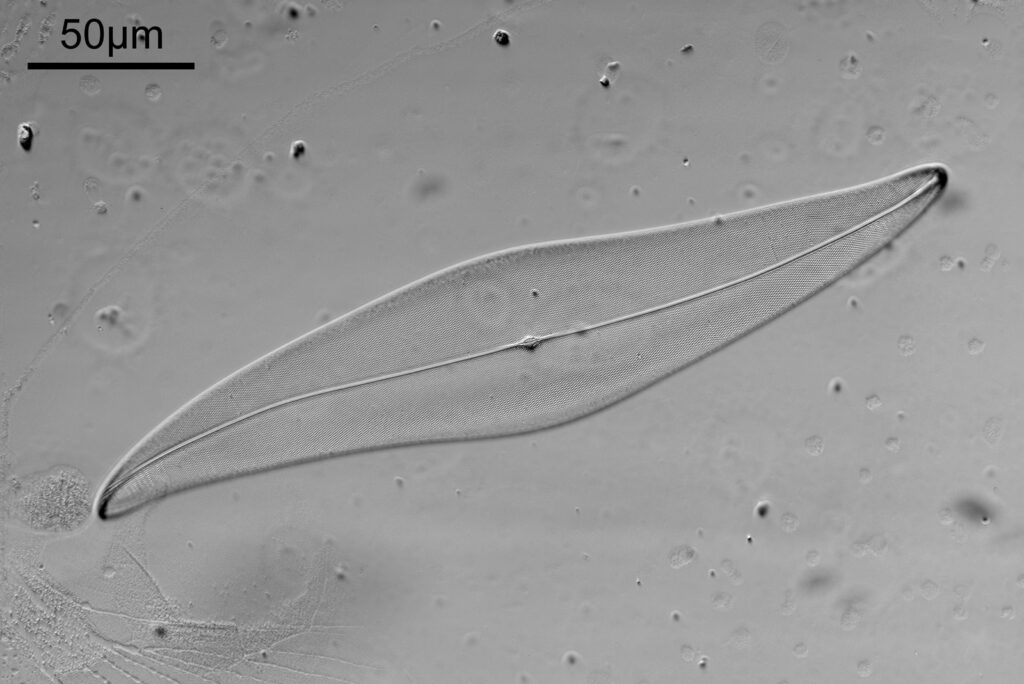
The sample is a slide by Charles Morgan Topping and likely dates to around 1860. I’ll show a picture of the slide at the end of the post. It has a thin section of coal on it and is described as “Trans sect[ion] of coal. Warwickshire”. The section of coal is about 1cm across and mounted under a coverslip. First a normal bright field image (using a 1x Olympus Splan FL NA 0.04 objective).
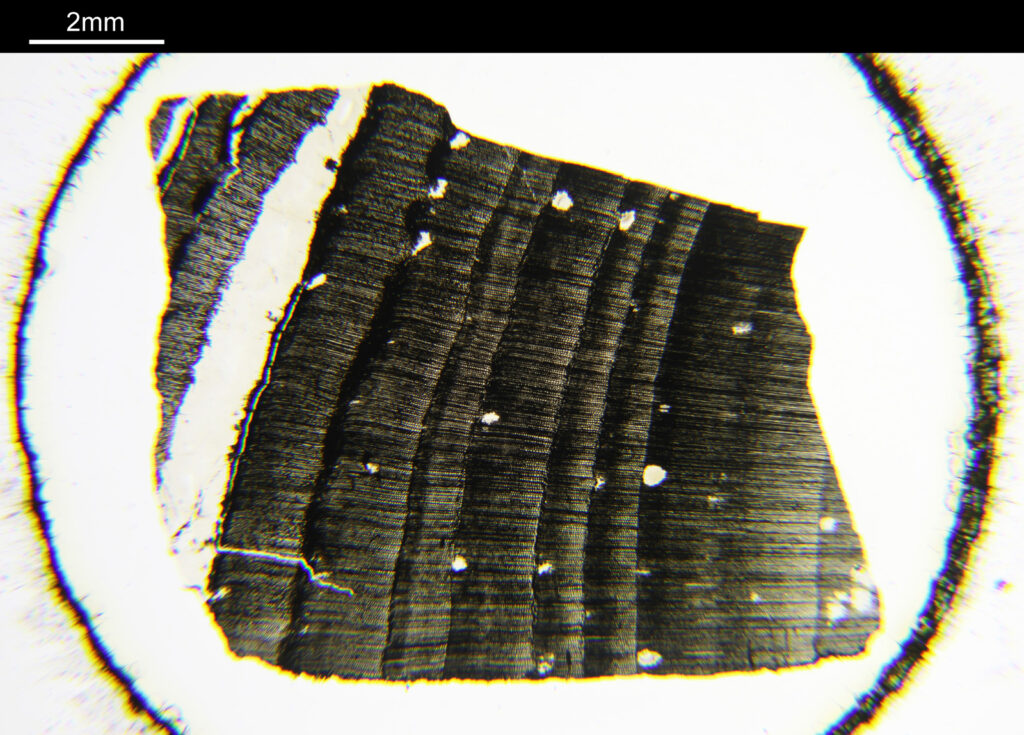
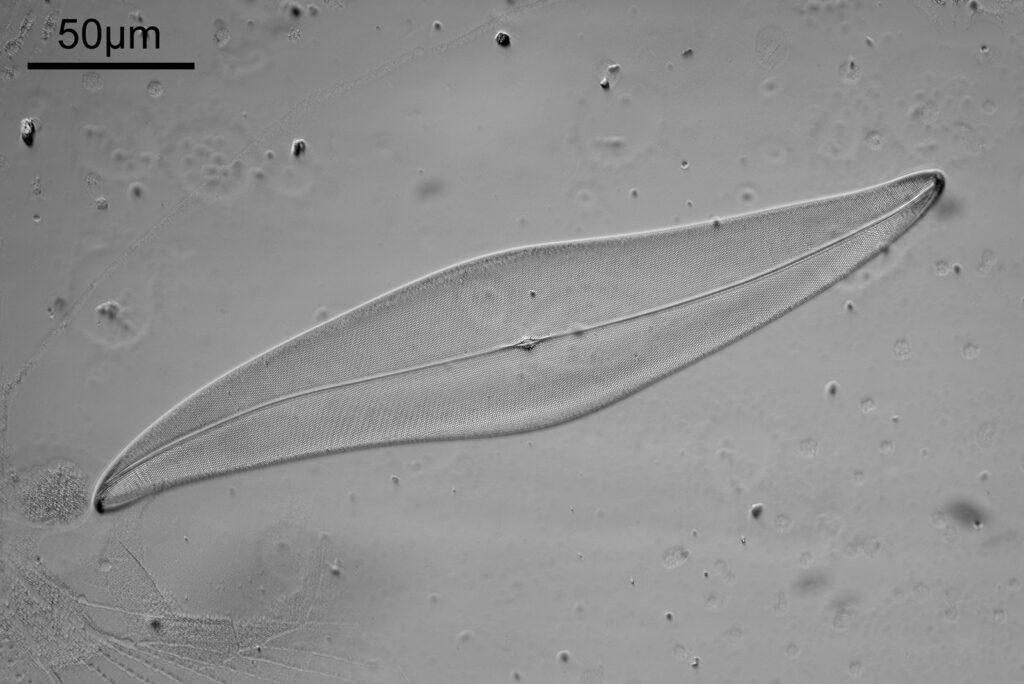
And now the same sample imaged using cross polarized light.
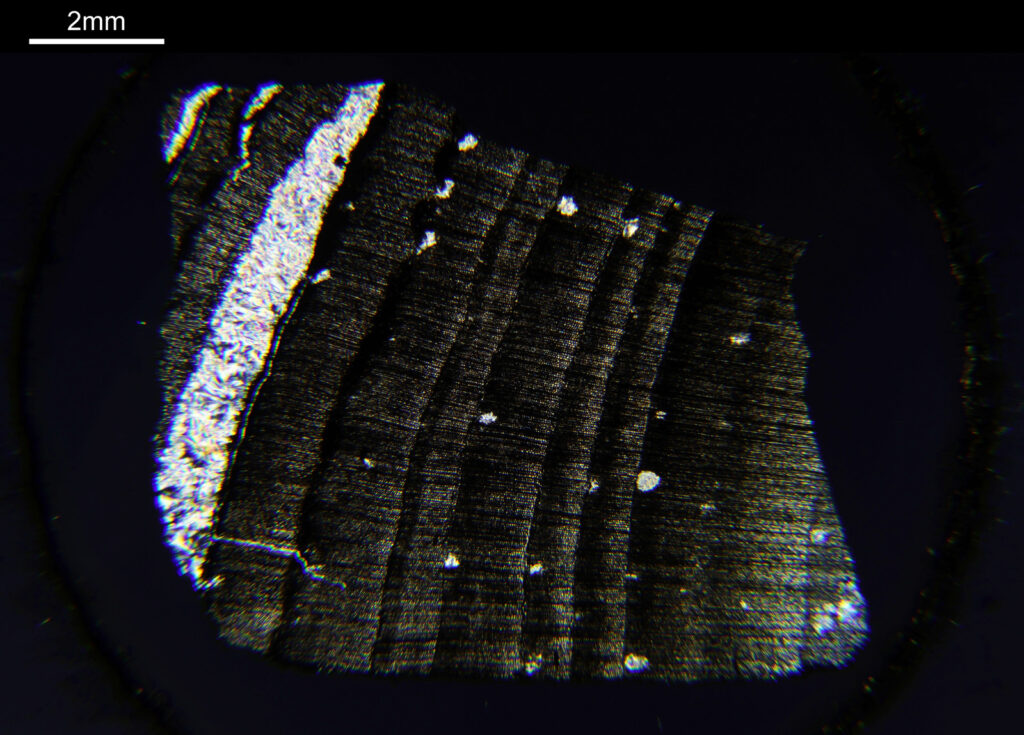
Note that to get the whole sample in the field of view, I had to remove some of the extension tubes from between the photoeyepiece and the camera. While the increases the field of view it does mean that the edges of the image (which wouldn’t normally be seen in the standard setup) are included. These parts of the images above have some pretty severe aberrations present which is why the edges are nowhere near as sharp as the middle of the image.
The cross polarized imaging sends the background very dark, and now crystalline material which is present becomes very colourful as it rotates the polarized light passing through it. At this scale, this is especially visible in the white band towards the left of the coal sample. This is where my knowledge of coal is getting a bit stretched. I think the dark bands running almost vertically in the images are growth rings from the original plant material – think tree rings.
Going in closer with a different objective now (a 2x Olympus Splan FL NA 0.08). All the images from now on will be cross polarized.
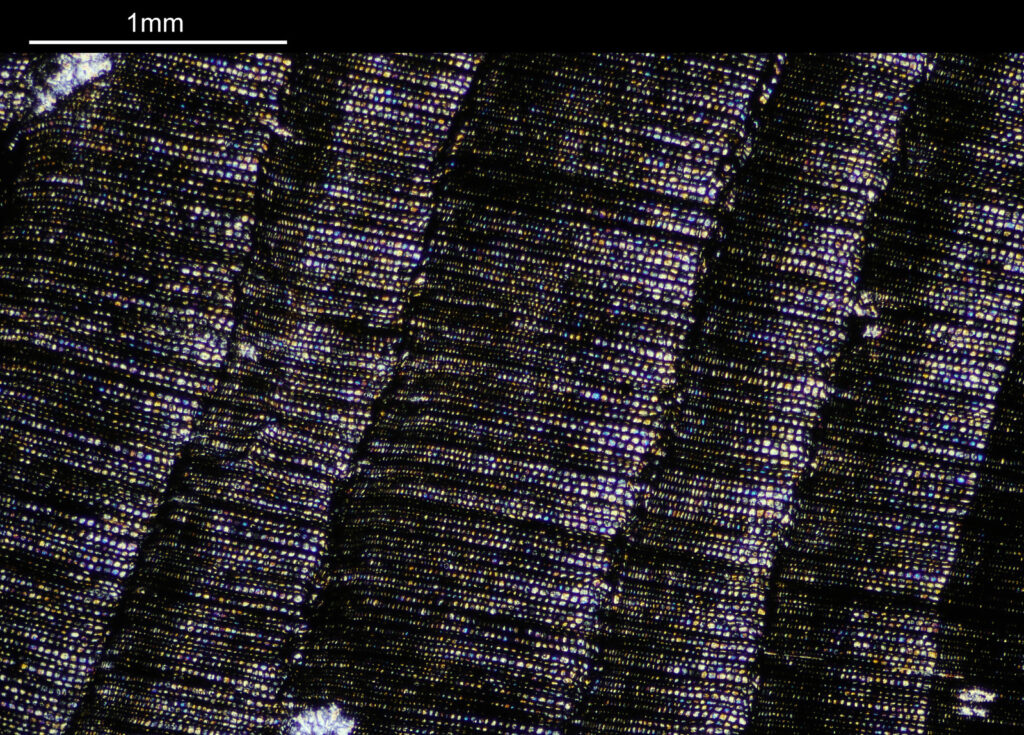
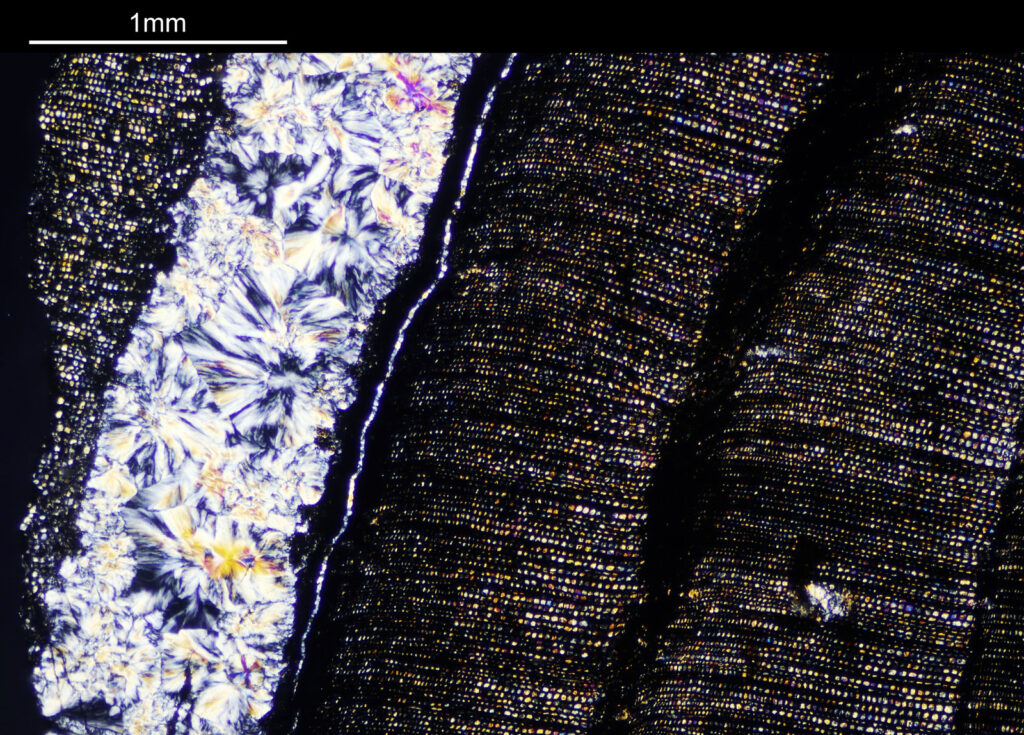
For the observant amongst you, you will have noticed that these images look more than 2x magnified compared to the images with the 1x objective. The extension tubes were put back in between the photoeyepiece and camera, having the effect of increasing the magnification (and getting rid of the aberrations at the end of the field of view). The dark bands running from top to bottom are now more clearly visible, and there are small ‘dots’ present as well in between the bands, which are coloured. The strongly coloured area towards the left of the image is the crystalline region shown towards to the left of the 1x images. This is very crystalline as can be seen from the colours present in the image.
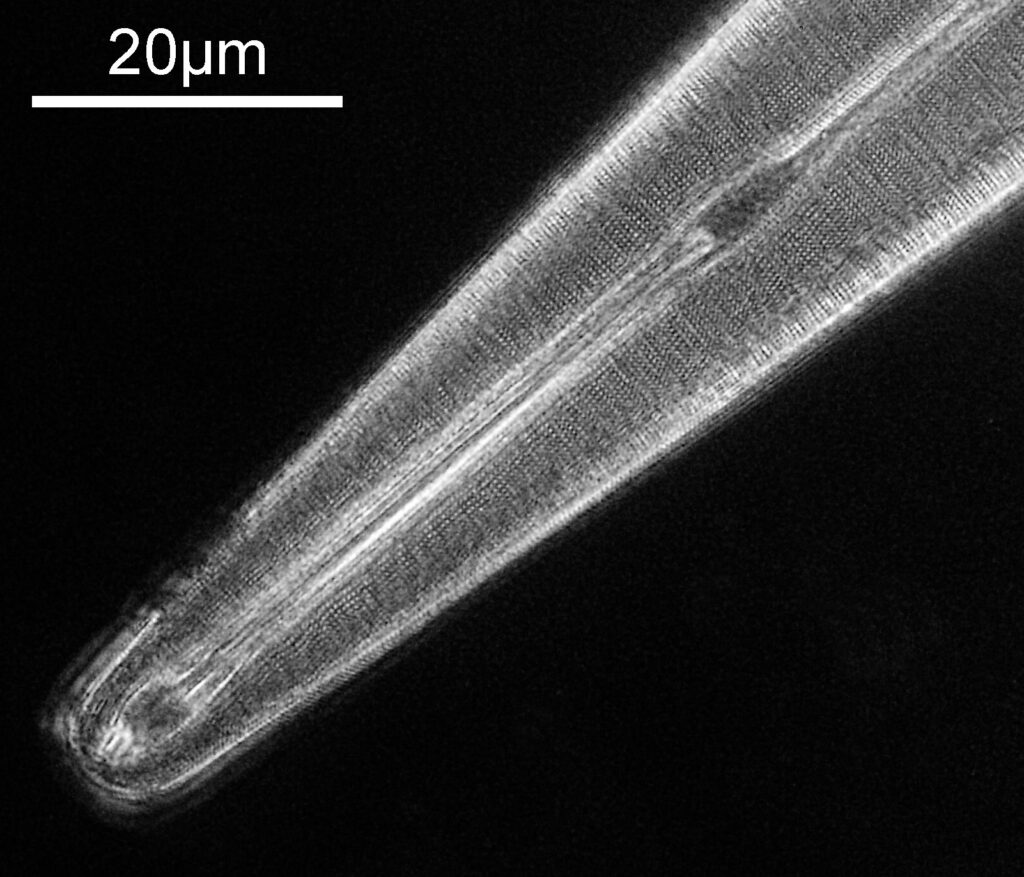
Going in even further with a 10x Olympus UVFL NA 0.4 objective, the ‘dots’ become more visible.
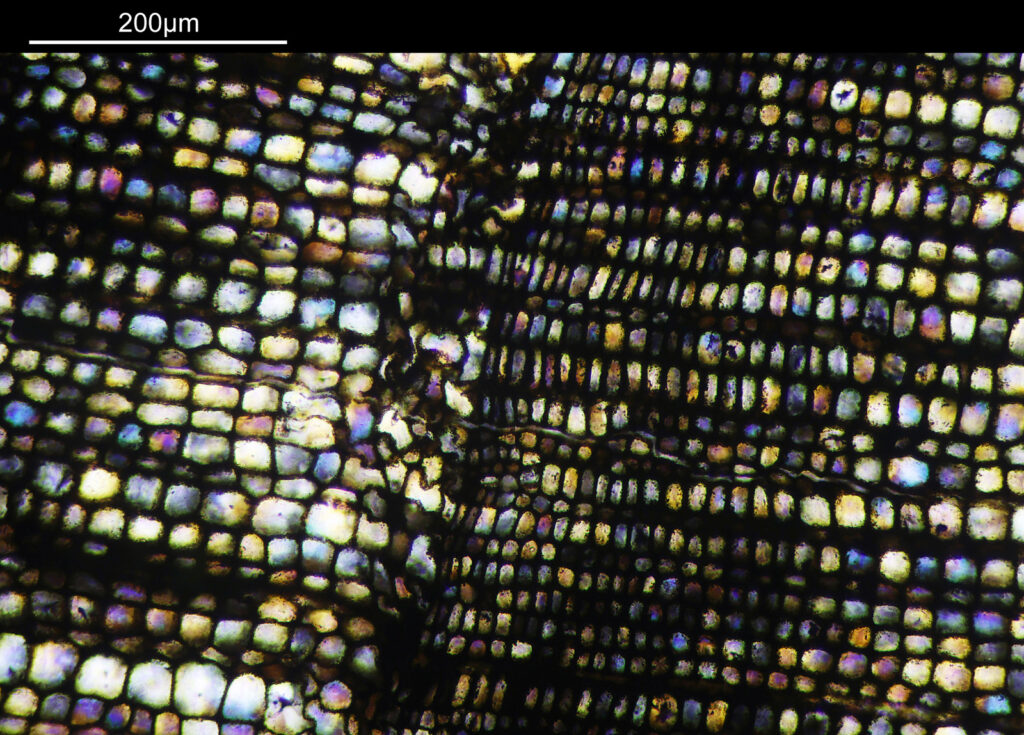

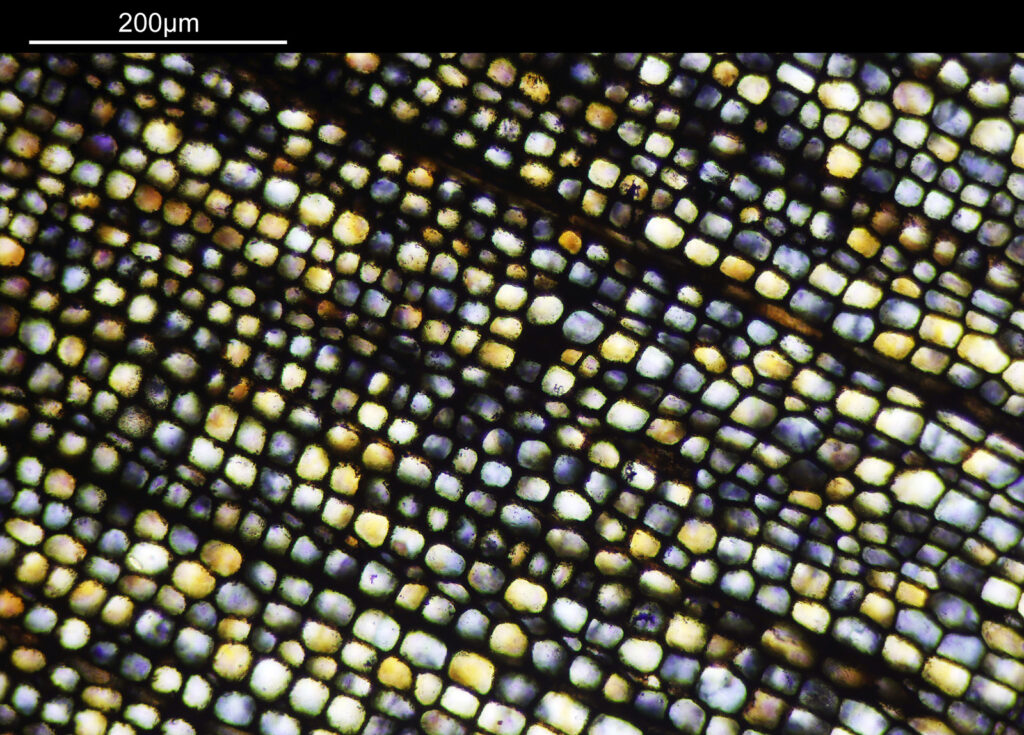
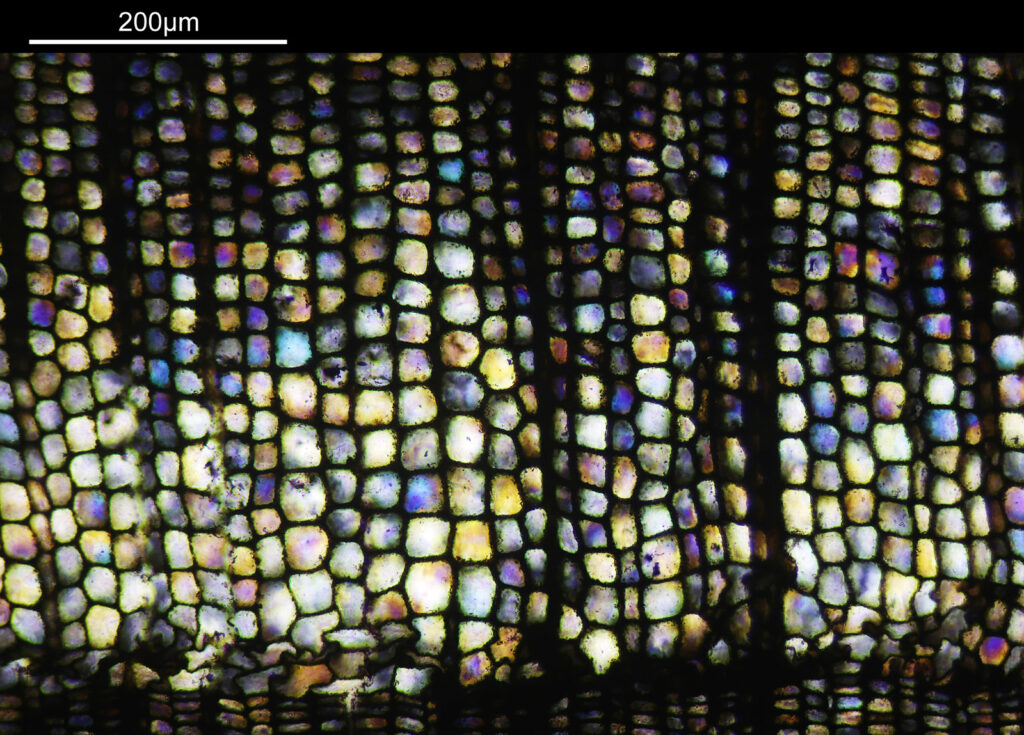


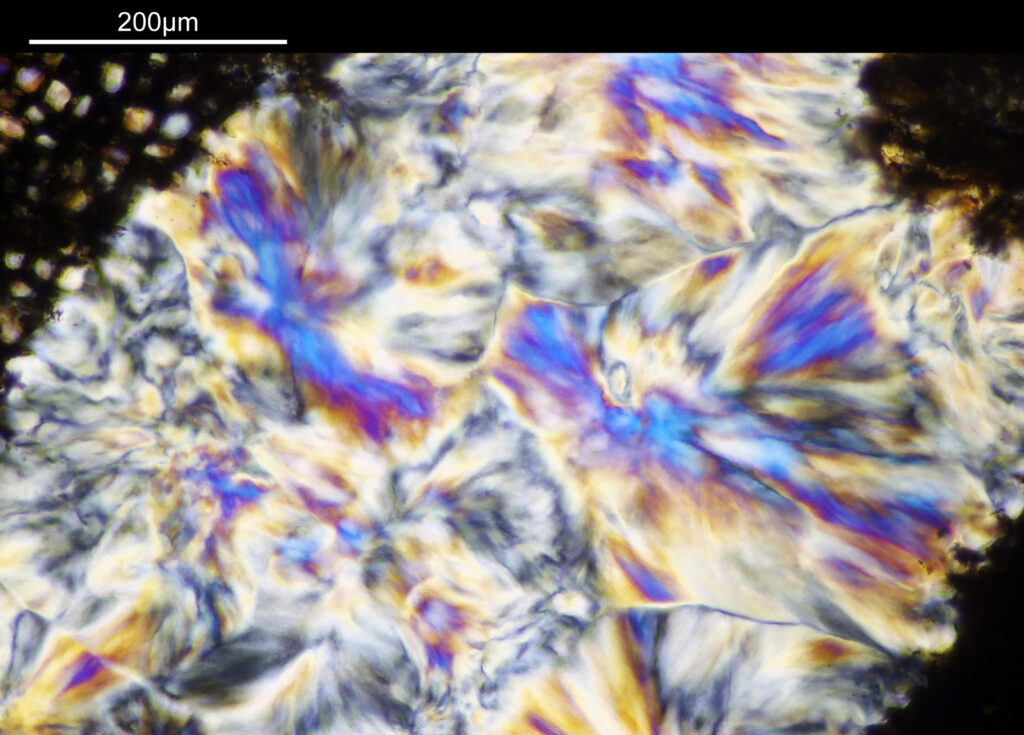
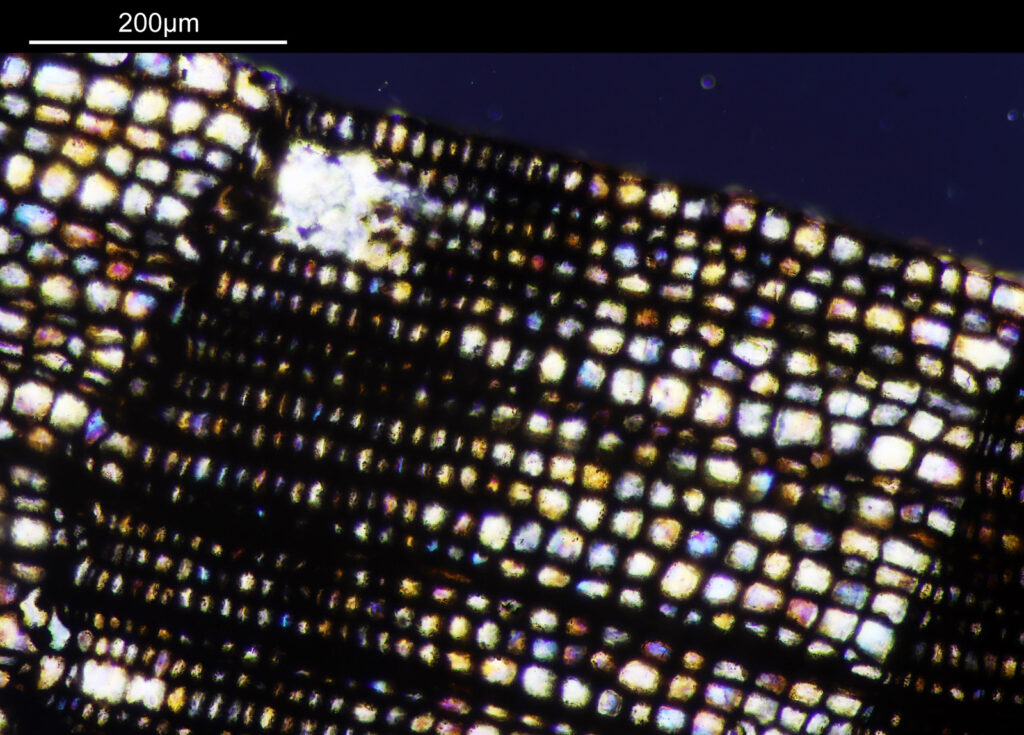
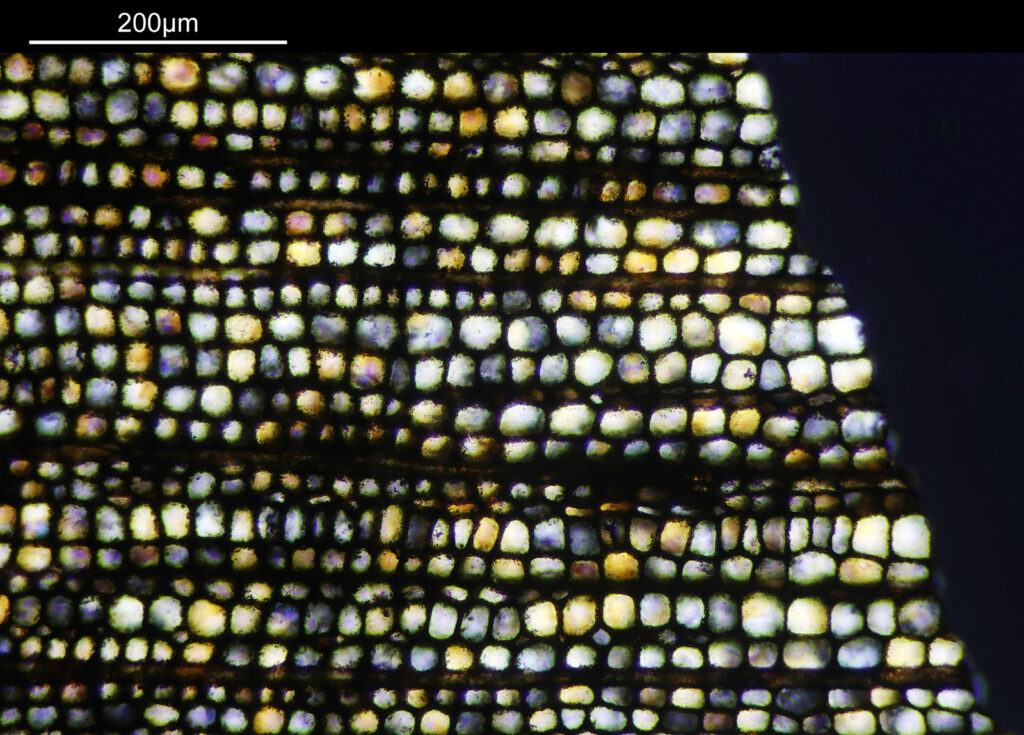
At this scale the ‘dots’ become little stained glass windows, taking on a kaleidoscope of colours. As I said before I think these areas were originally cells in the plant matter when it was alive. These have then become filled with crystalline mineral during the fossilization process. The slide says ‘Warwickshire’ on it, and if so the coal fields of Warwickshire date to 300-350 million years ago. If these features were originally cells in the plant, then they are 300-350 million year old cells.
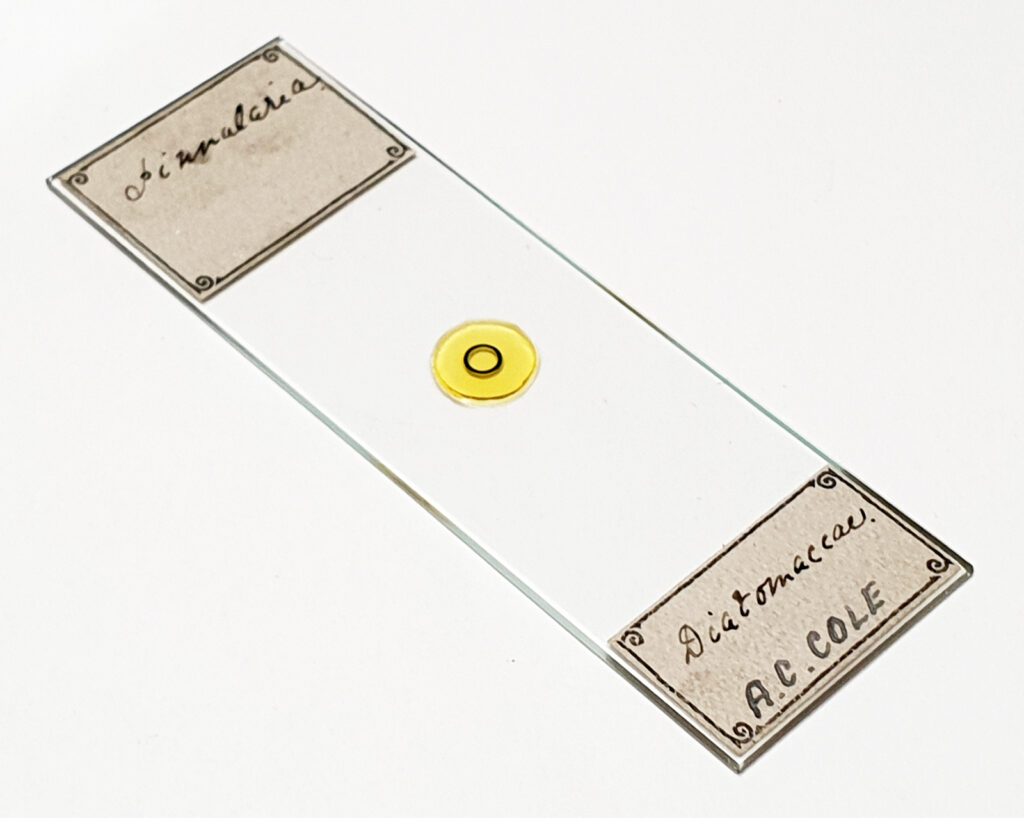
Some additional information. Here’s the slide.

I’ve been told this was made by Charles Morgan Topping and dates to around 1860. There’s more on Topping here.
Also, the two low magnification objectives – Olympus 1x and 2x SPlan Fl.

Olympus 1x and 2x SPlan Fl objectives
As always, thanks for reading, and if you’d like to know more about this or other aspects of my work I can be reached here.