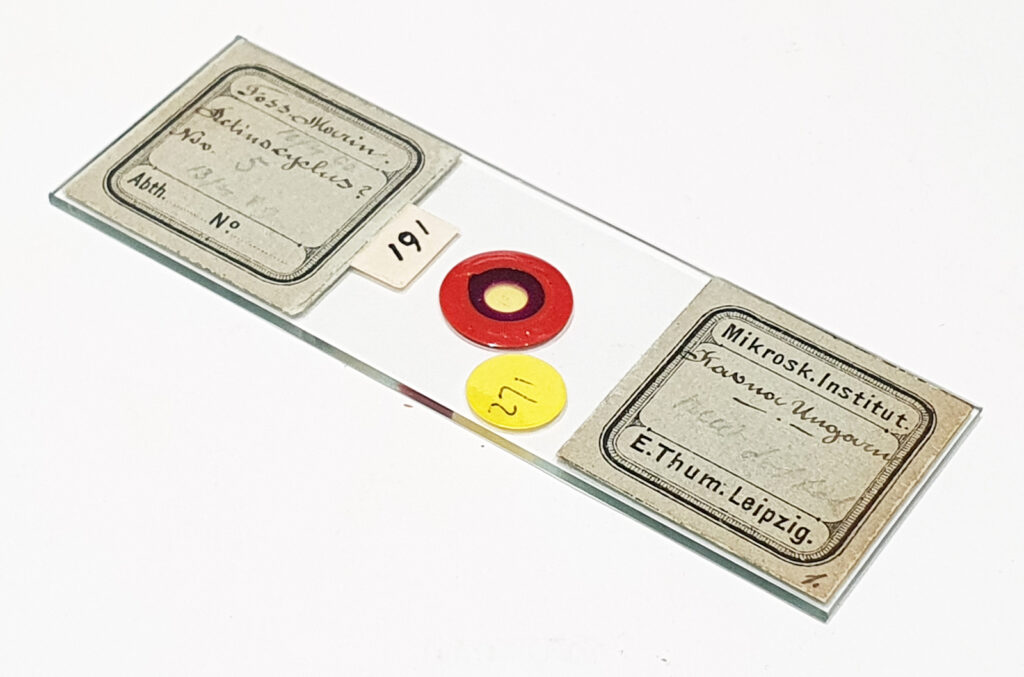
It’s no secret that I like the old diatom microscope slides. They can be very well made and provide very photographable diatoms often for much lower prices than modern prepared slides. The one I’m sharing today was by Samuel Henry Meakin and was made in 1945. It is a diatom test slide with examples of a variety of different species. Images were taken on my modified Olympus BHB microscope, and using a range of different objectives (4x Zeiss Planapo, 10x Olympus UVFL, 20x Olympus Splan, and 60x Olympus Splan Apo). Lighting was from below via an Olympus Aplanat Achromat condenser (straight bright field and oblique), and was 450nm LED light. Photoeyepiece was an Olympus 2.5x NFK, and the camera a monochrome converted Nikon d800. No stacking was done for these. Images are shown at reduced resolution where the originals were >1600 pixels across (most of them).
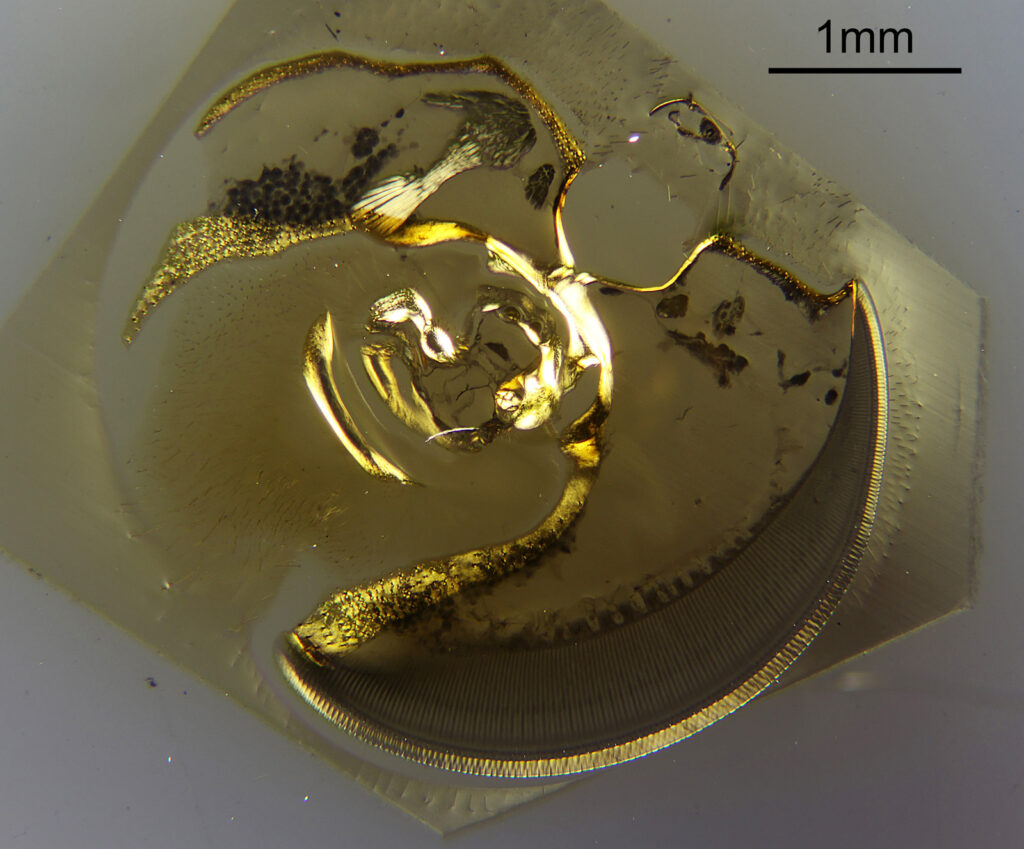
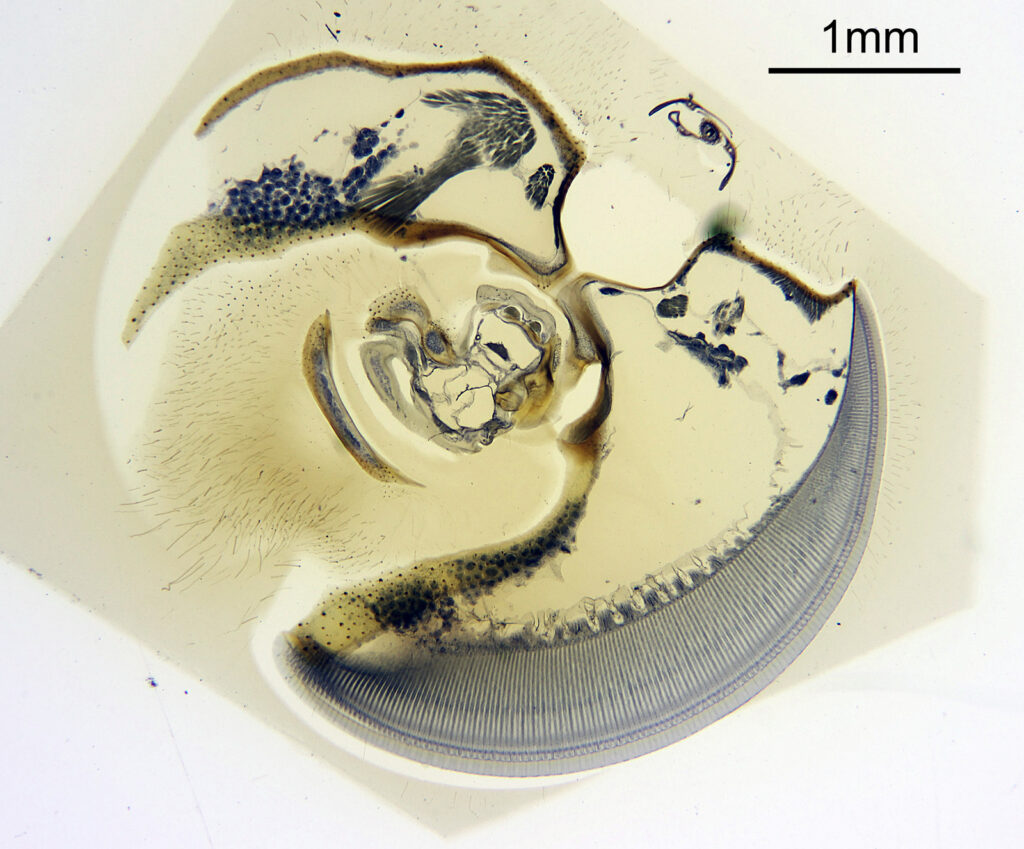
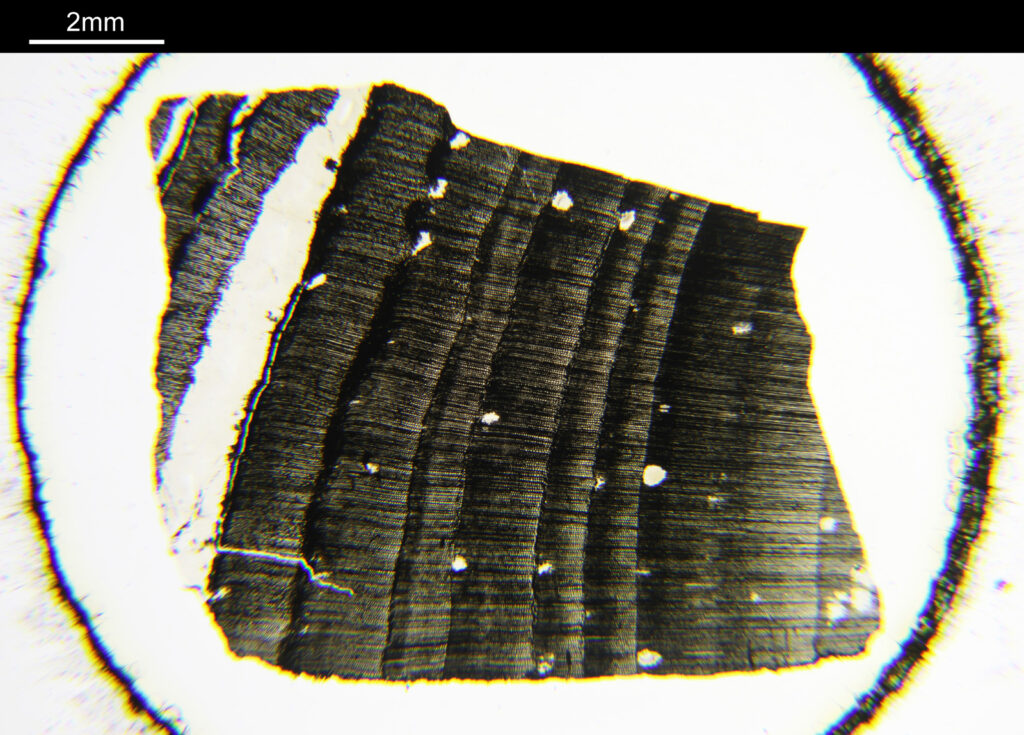
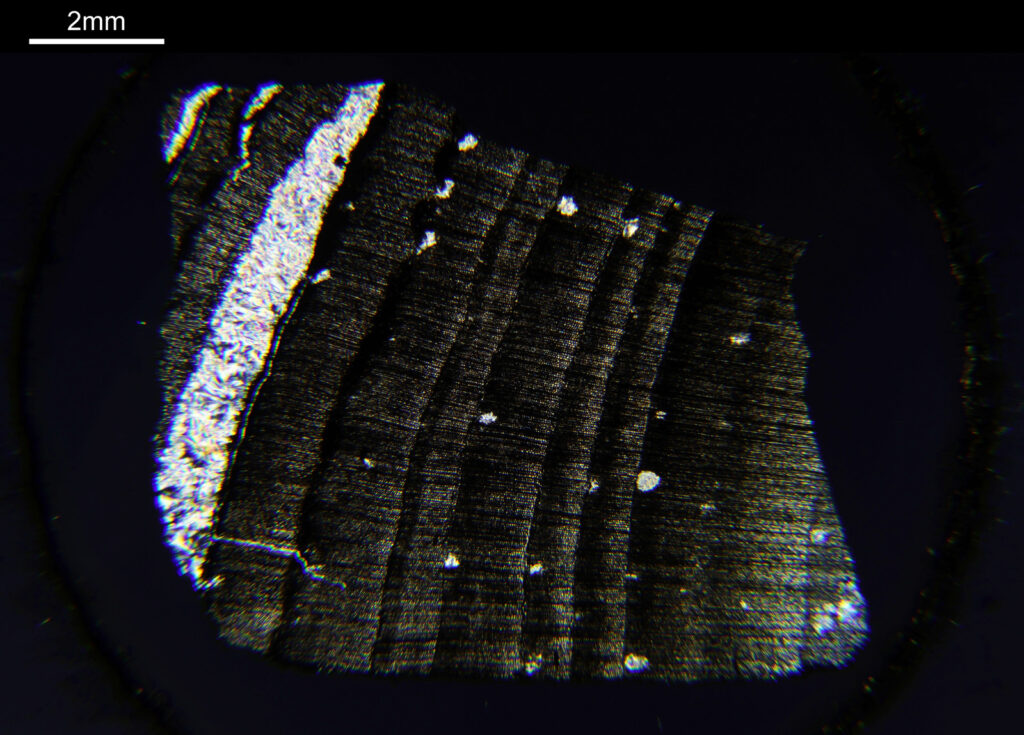
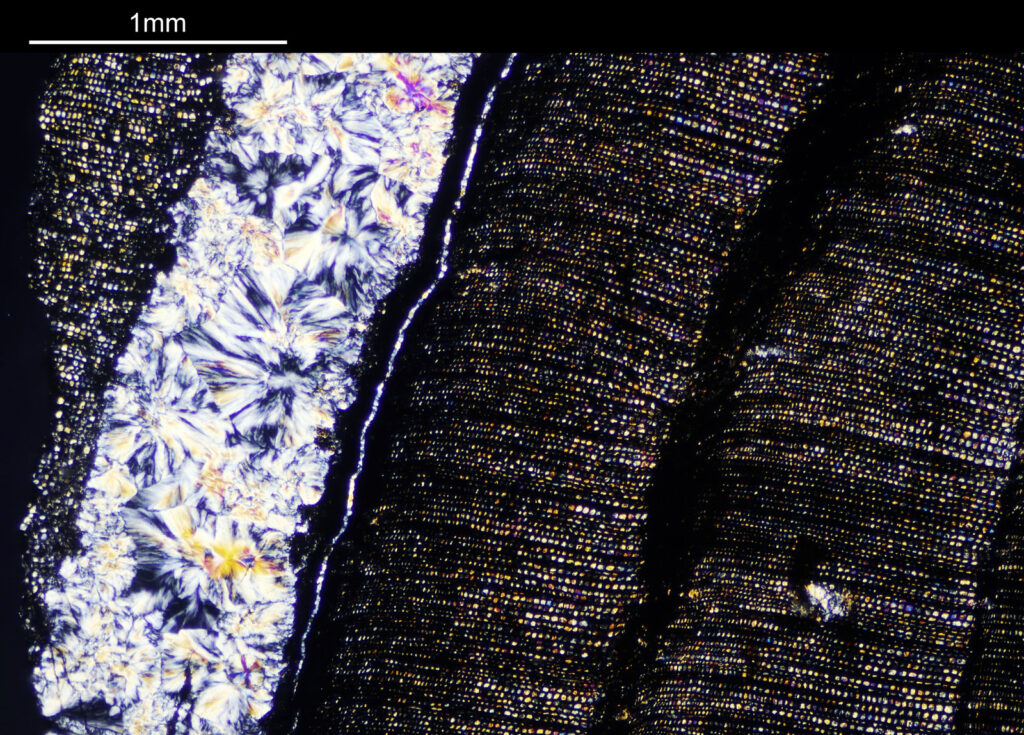
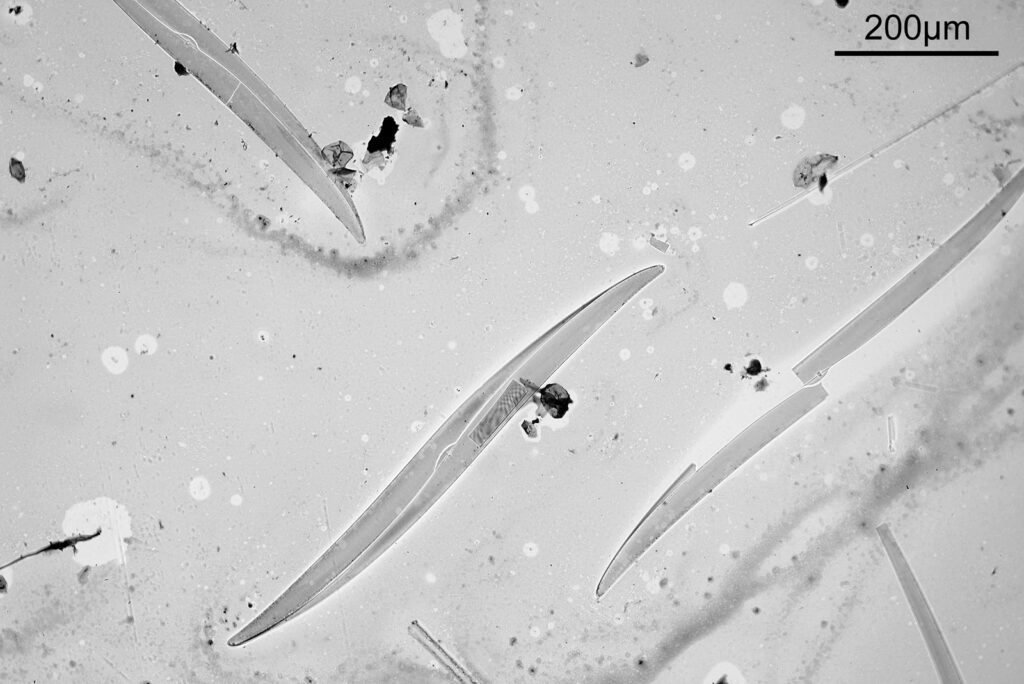
To start with, a low magnification image of the arrangement (4x objective).
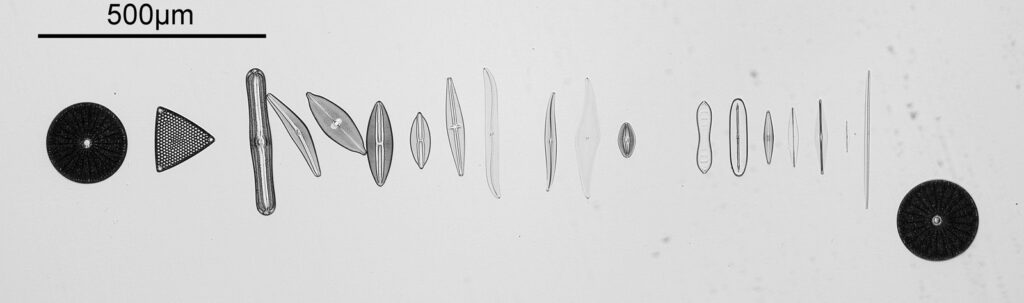
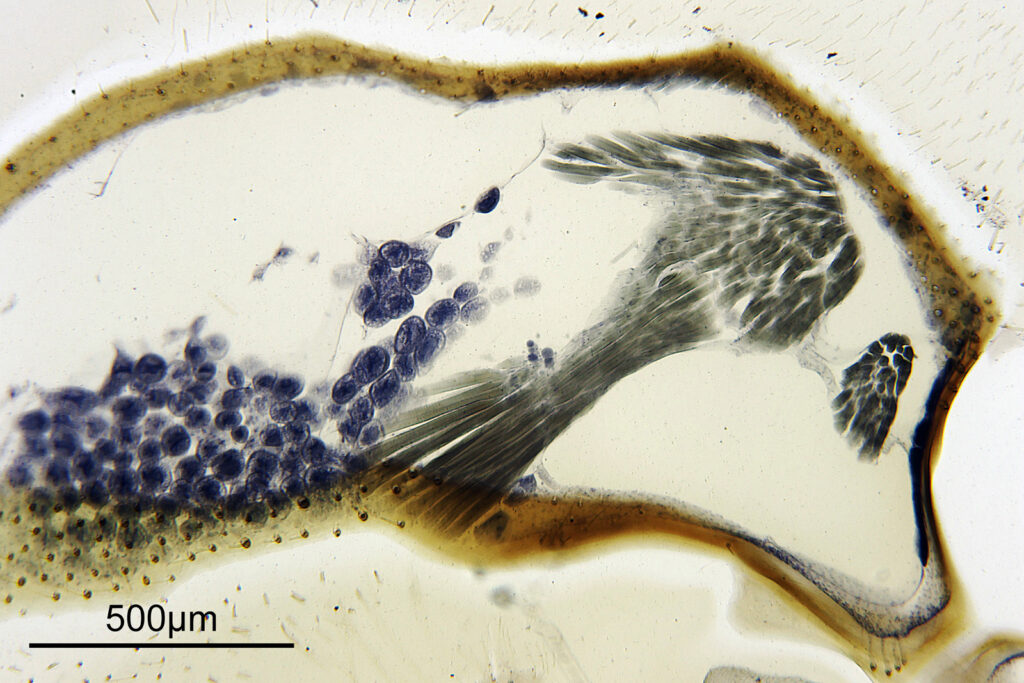
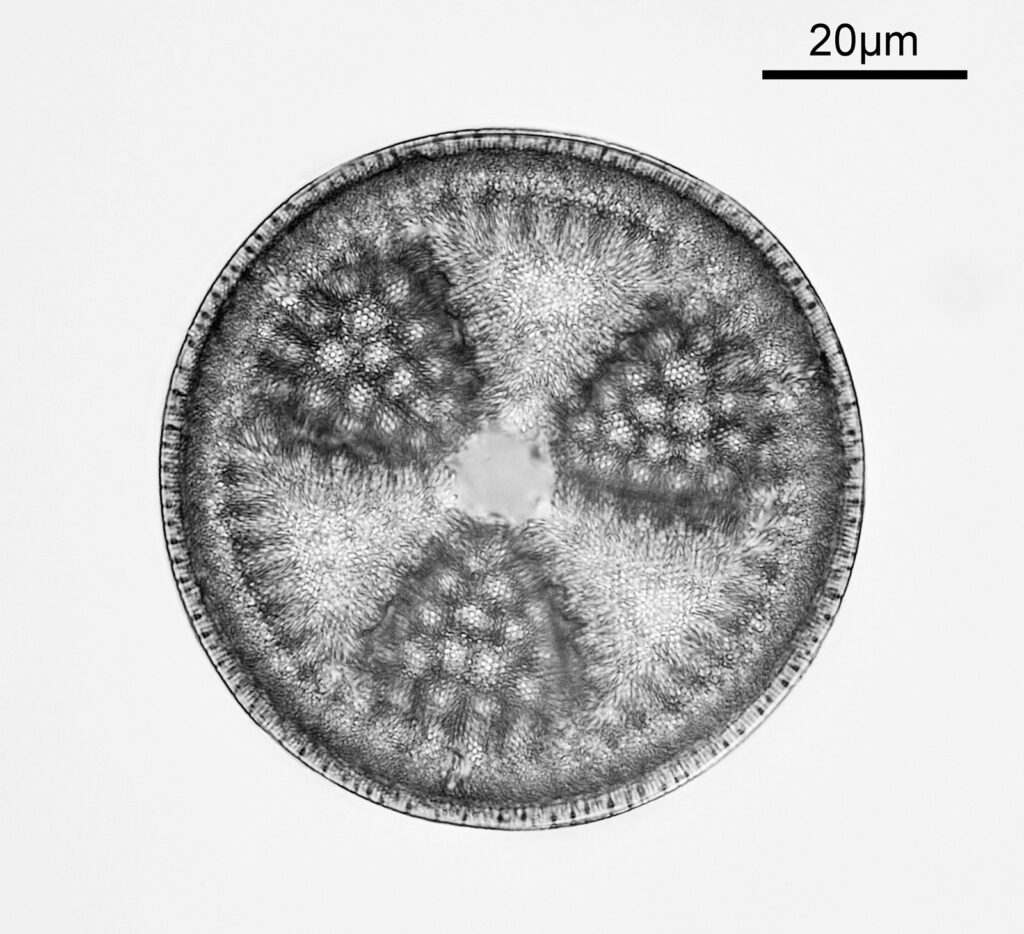
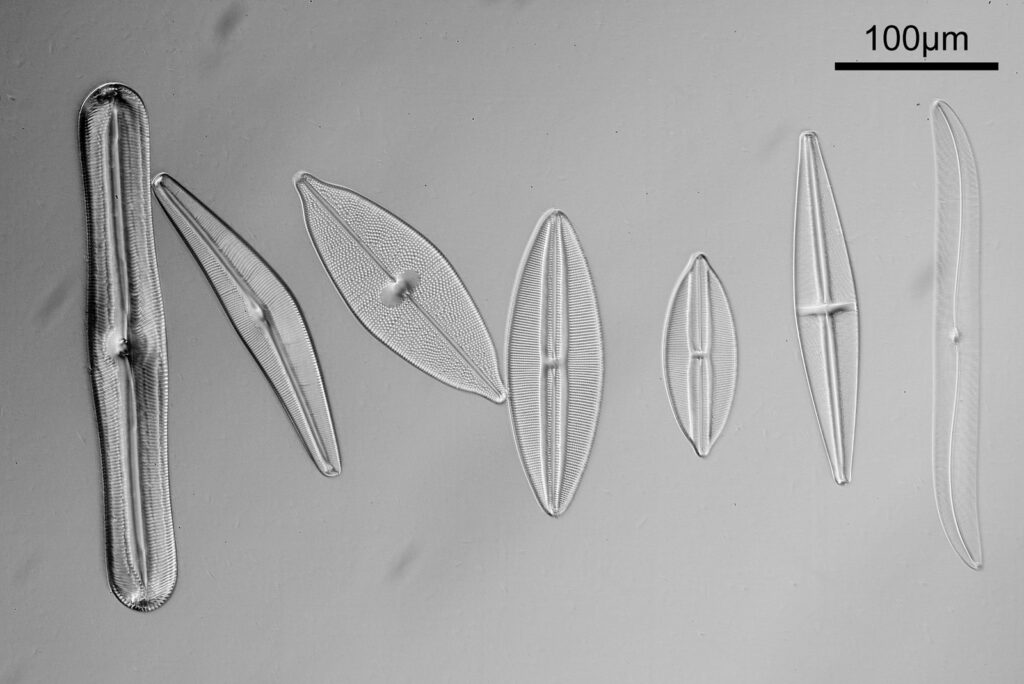
The test diatoms are bounded at the left and right by large circular diatoms. Using 2 circular diatoms like this was often done by the slide maker Eduard Thum to help with locating the samples on the slide. They are arranged in a linear fashion, although there has been a little movement of some of them. The slide is shown below.

What is great here is that Meakin has labelled the diatoms present (although not the circular ones on either end). They read as follows, going from left to right on the slide;
- Triceratium favus
- Pinnularia nobilis
- Cymbella gastroides
- Navicula maculata
- Navicula lyra coarse
- Navicula lyra fine
- Stauroneis phoenicenteron
- Pleurosigma balticum
- Pleurosigma attenuatum
- Pleurosigma angulatum
- Navicula smithii
- Cymatopleura solea
- Navicula lewisiana (also known as Frikea lewisiana)
- Brebissonia boekii
- Amphipleura lindheimeri
- Navicula rhomboides (Cherryfield)
- Navicula rhomboides fine
- Nitzschia singalensis
Some of these took some tracking down to get the details right as I struggled with the hand writing at times (especially for the less common ones). Overall the quality of the slide was very good, and the diatoms were intact. This is one reason why these types of test slides can be very nice – lots of intact, well prepared diatoms, great for imaging.
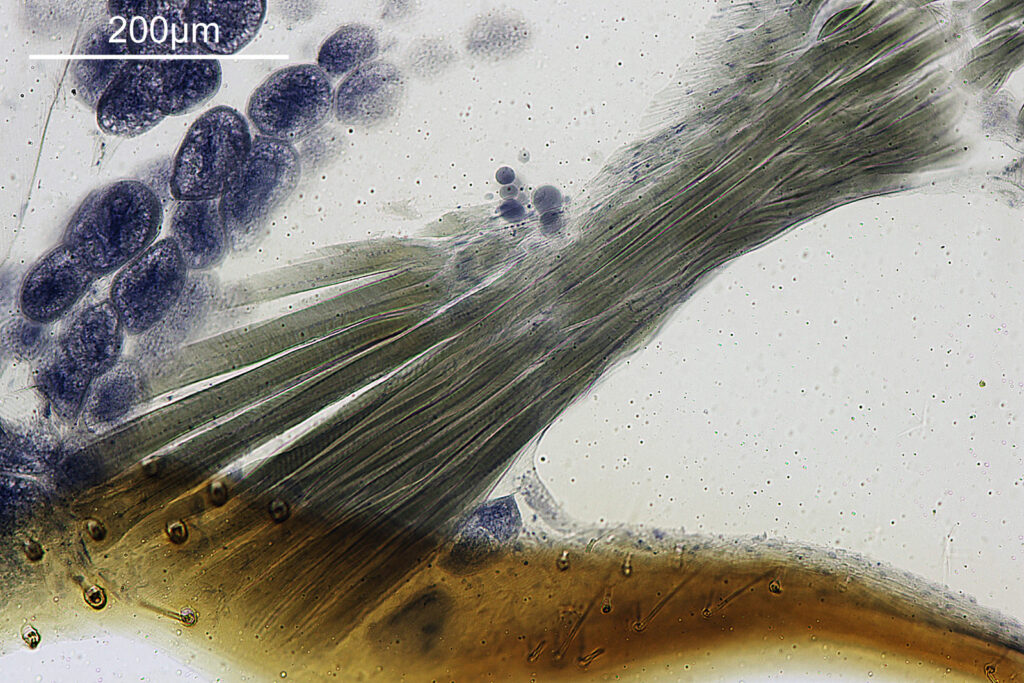
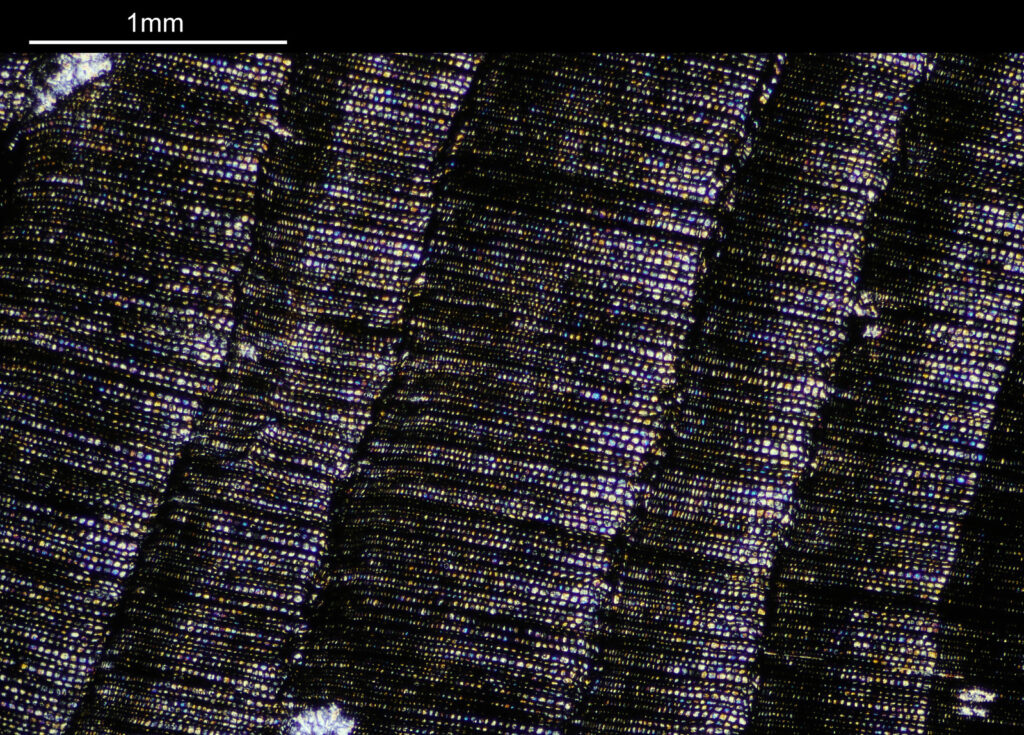
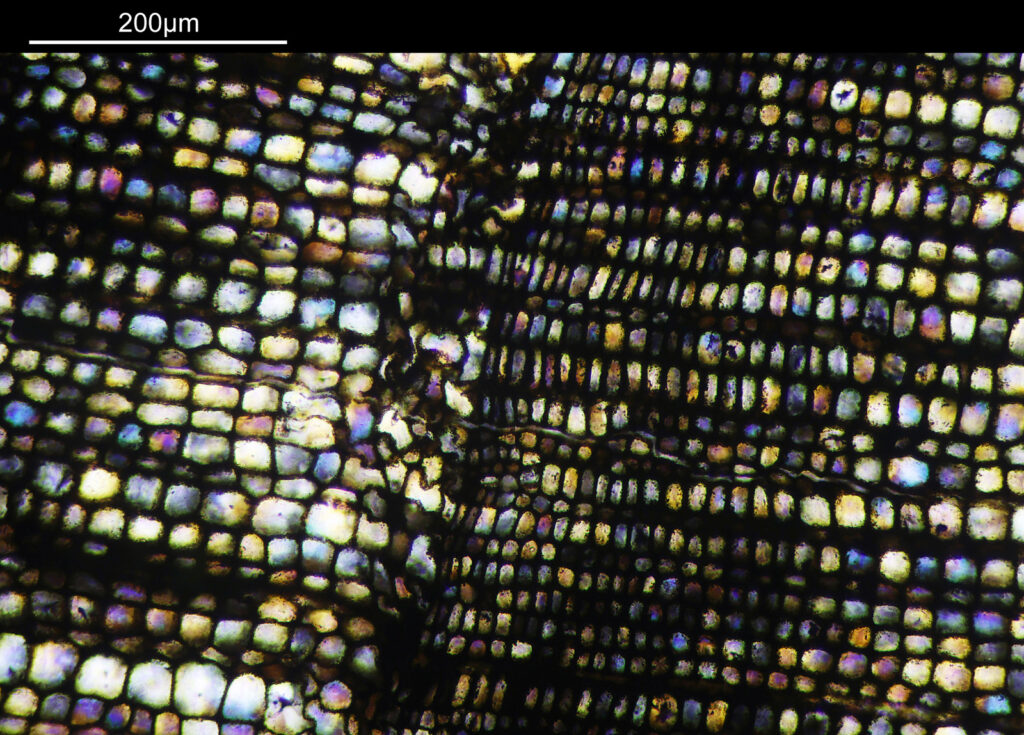
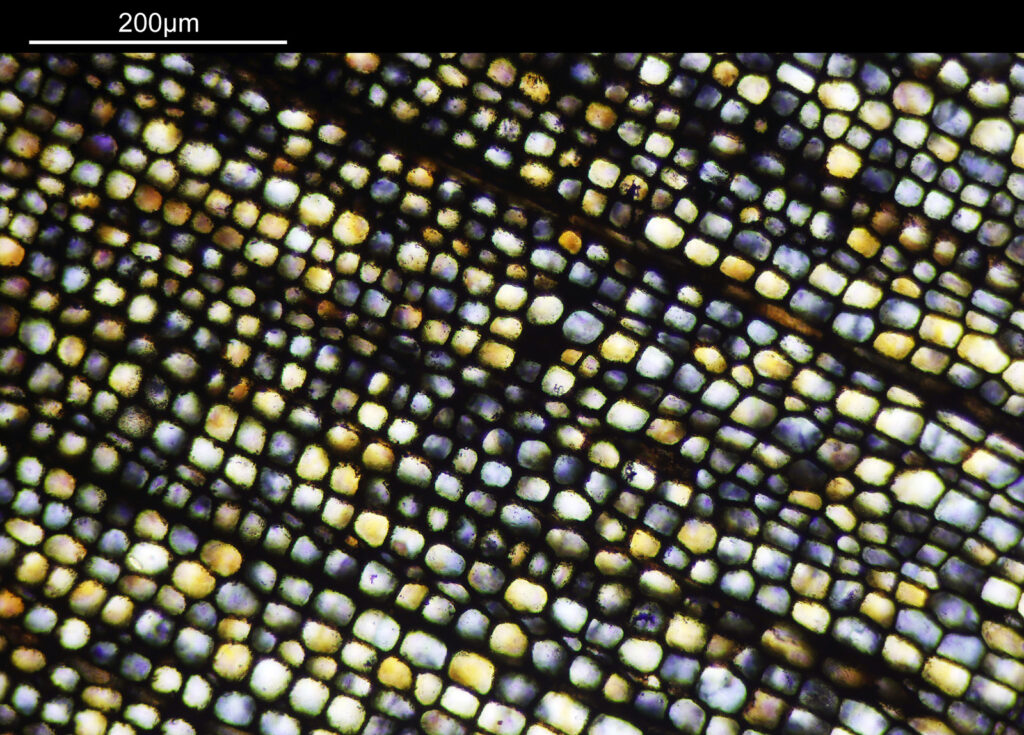
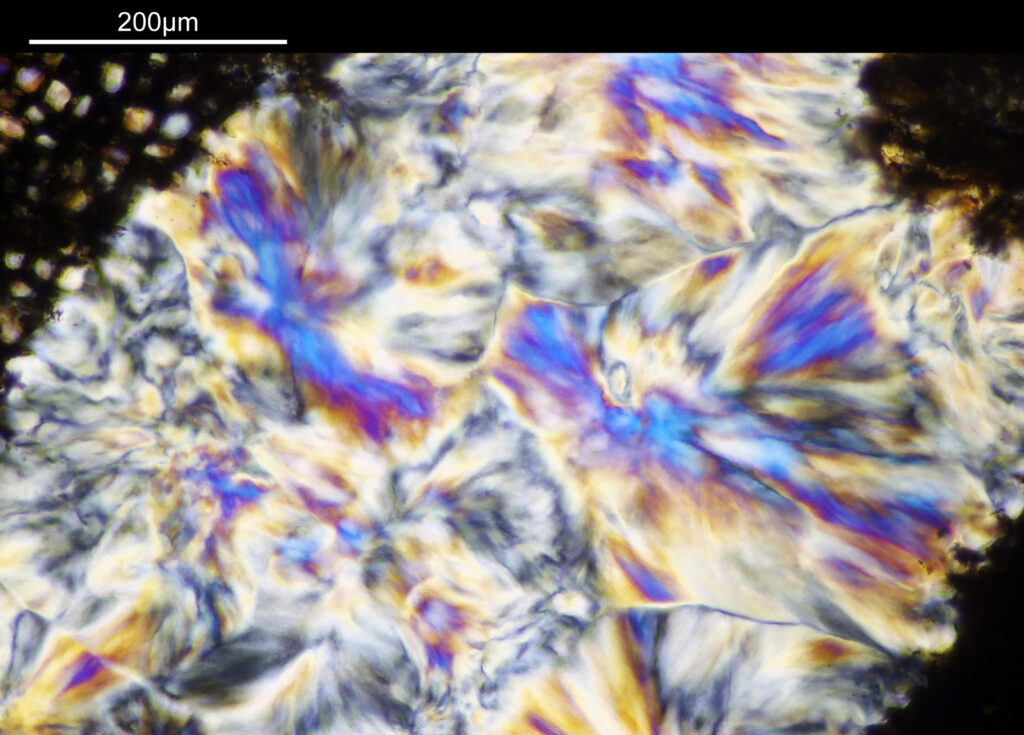
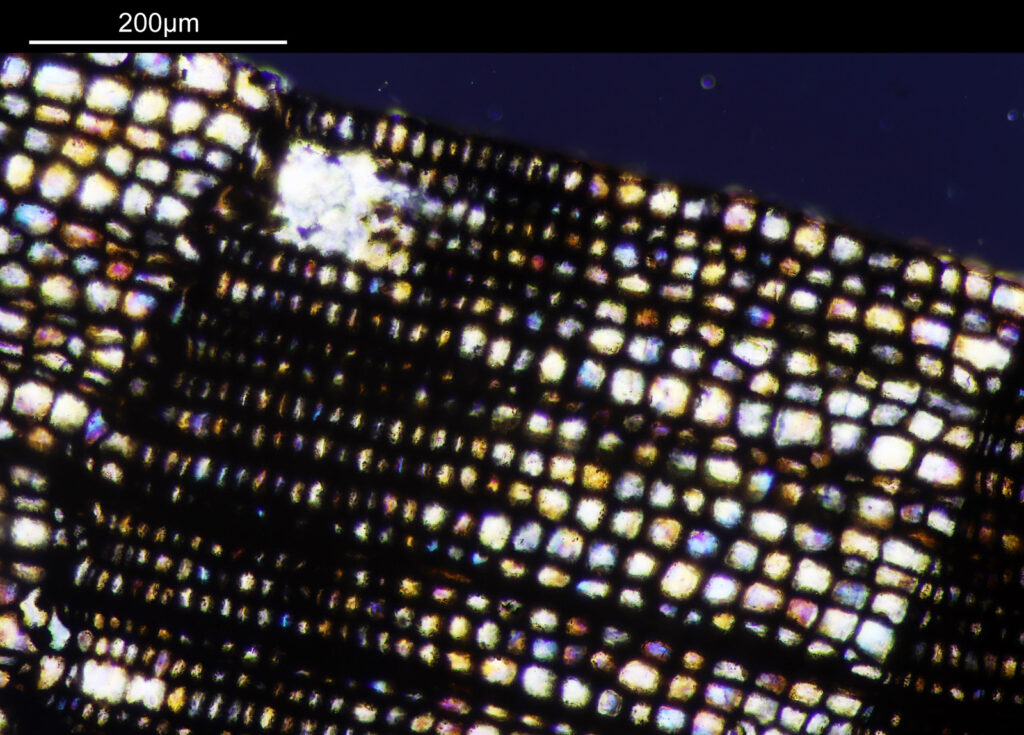
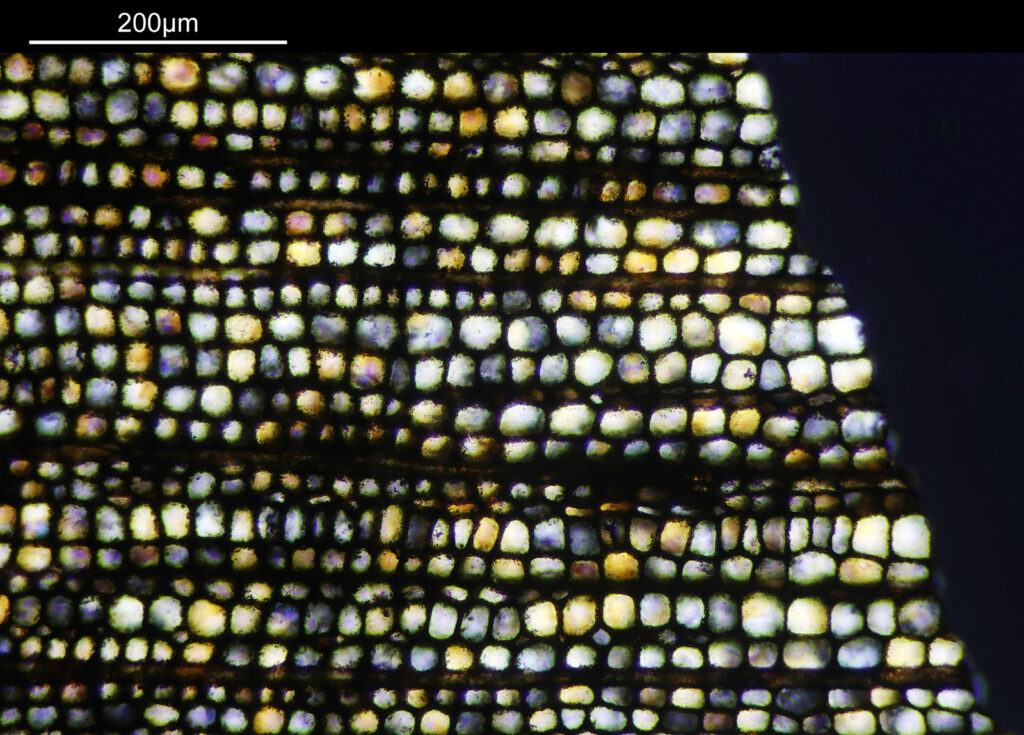
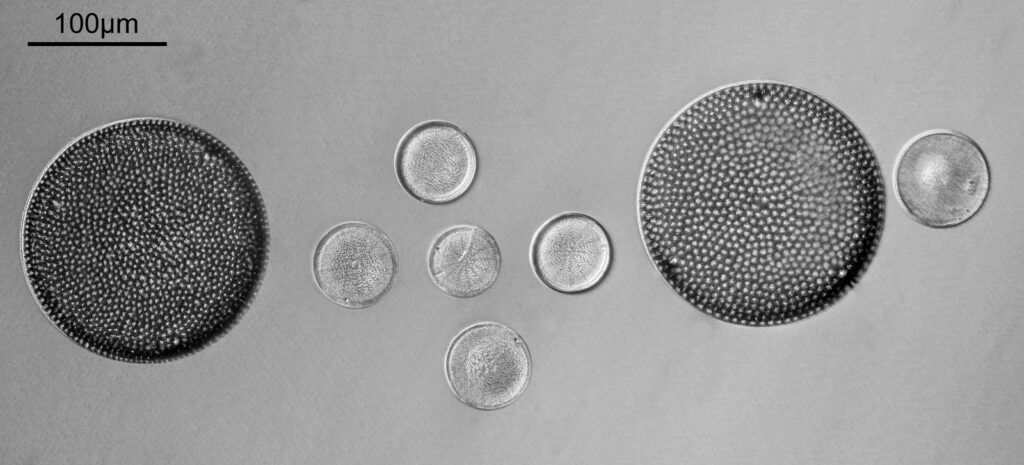
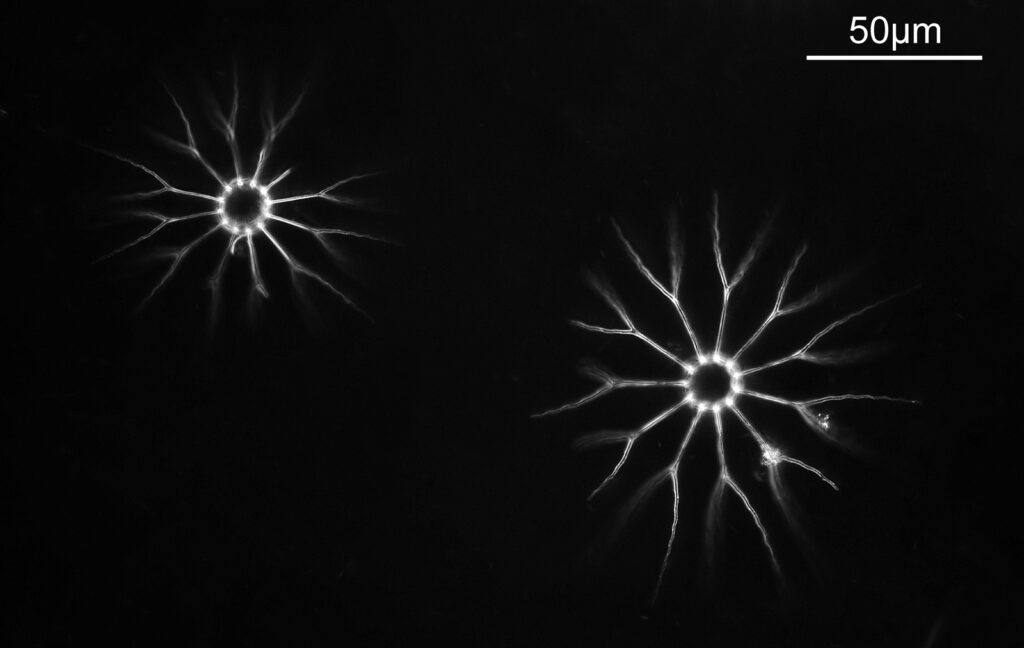
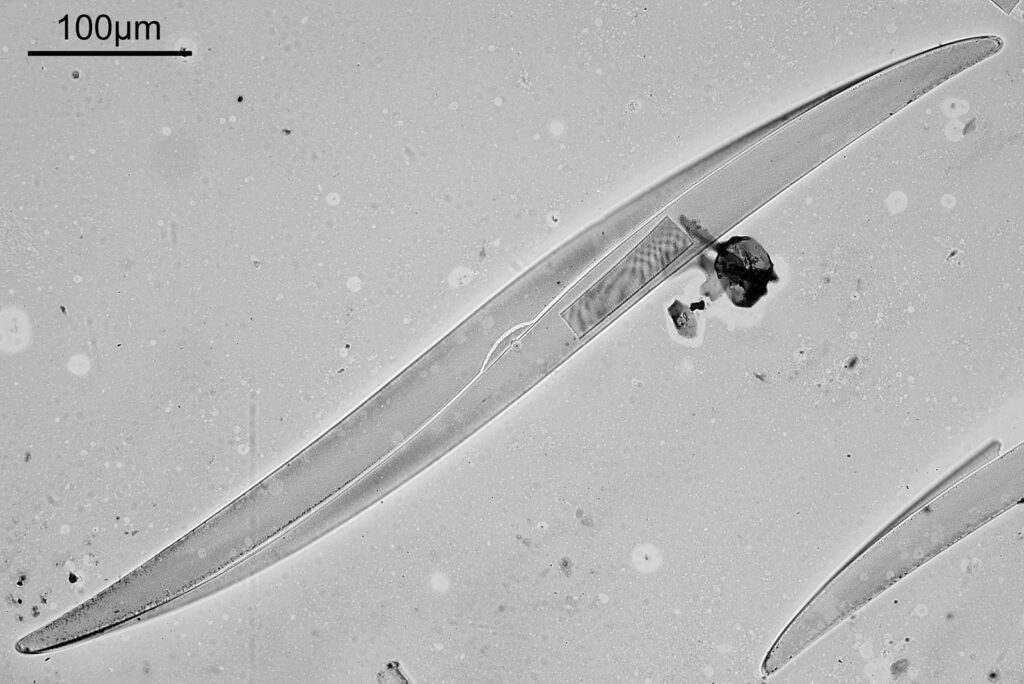
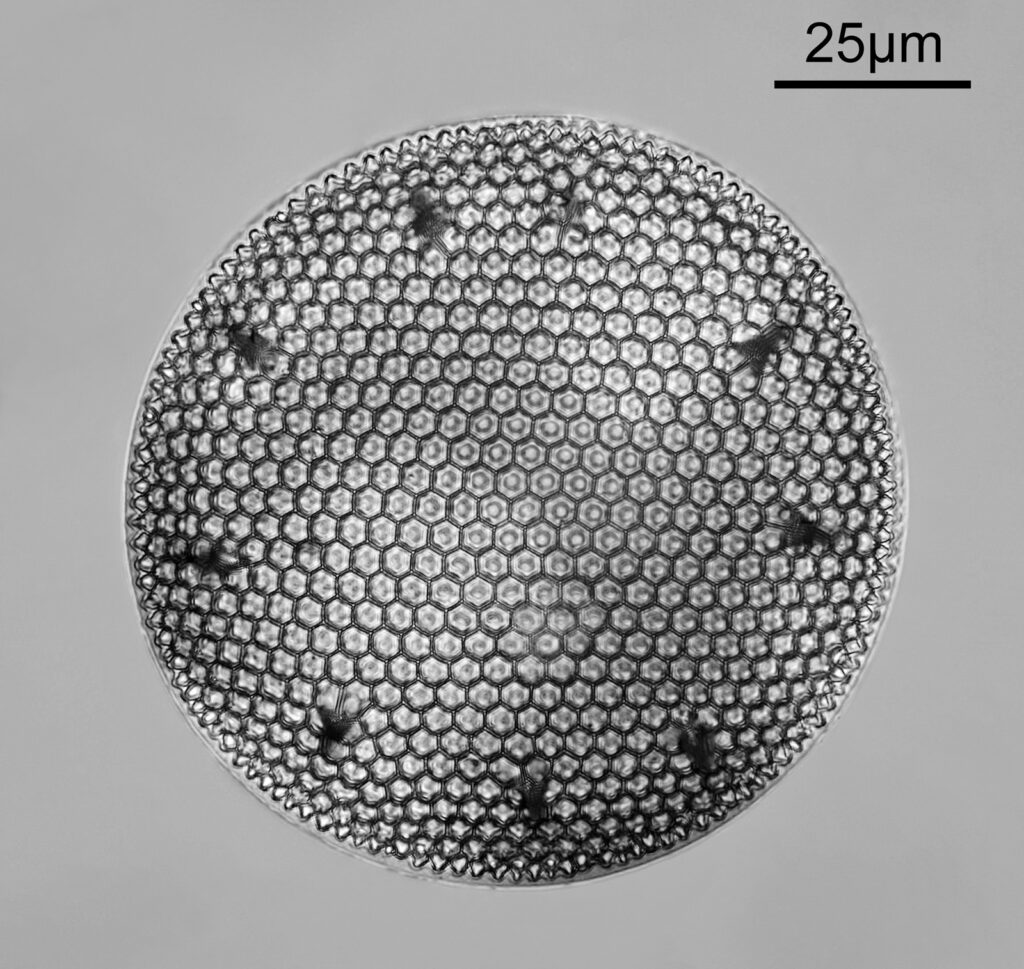
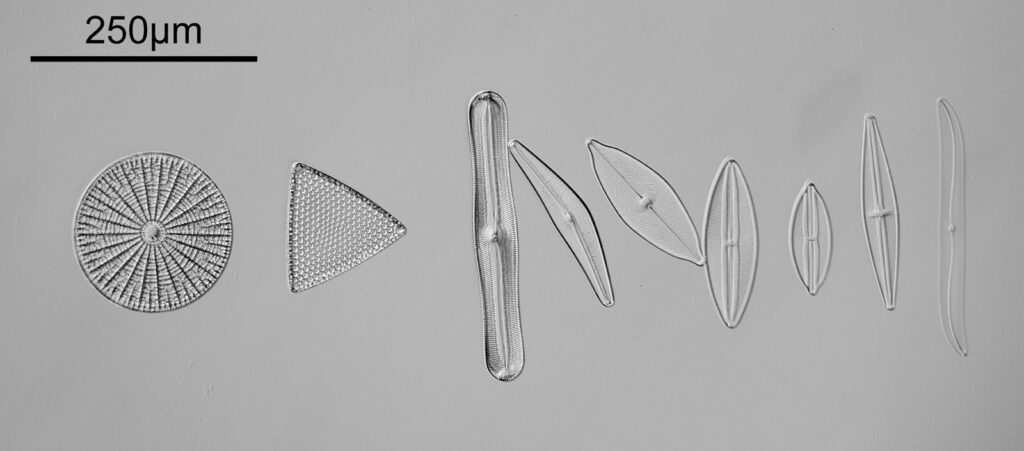
Going in closer, here are 2 images done with the 10x objective, oblique lighting.

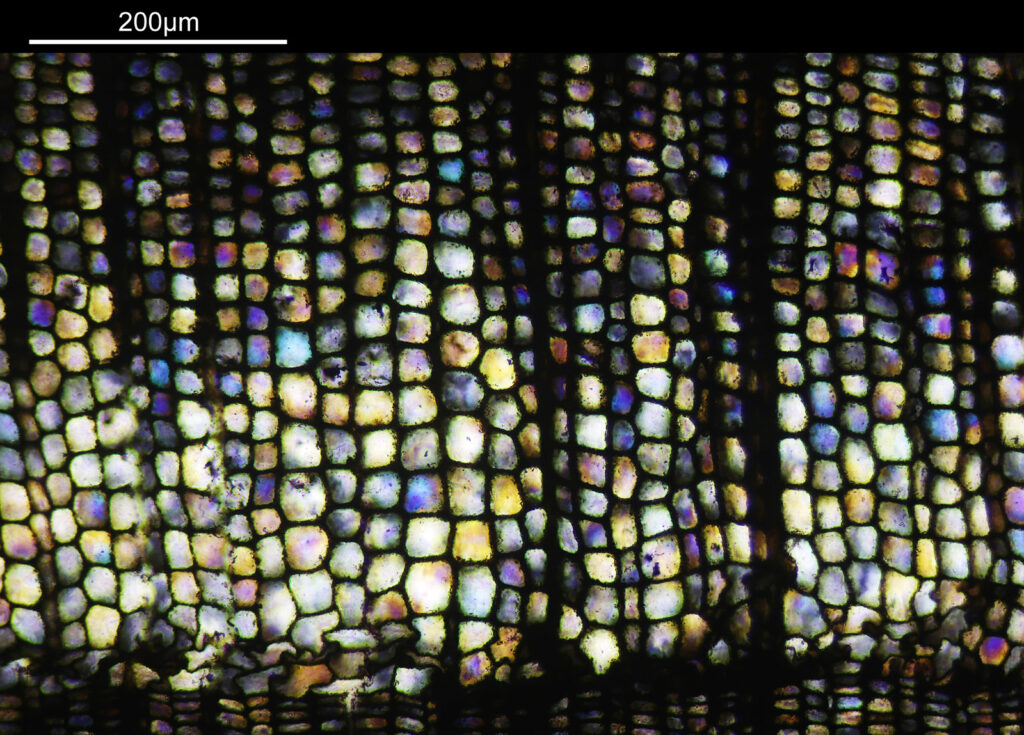
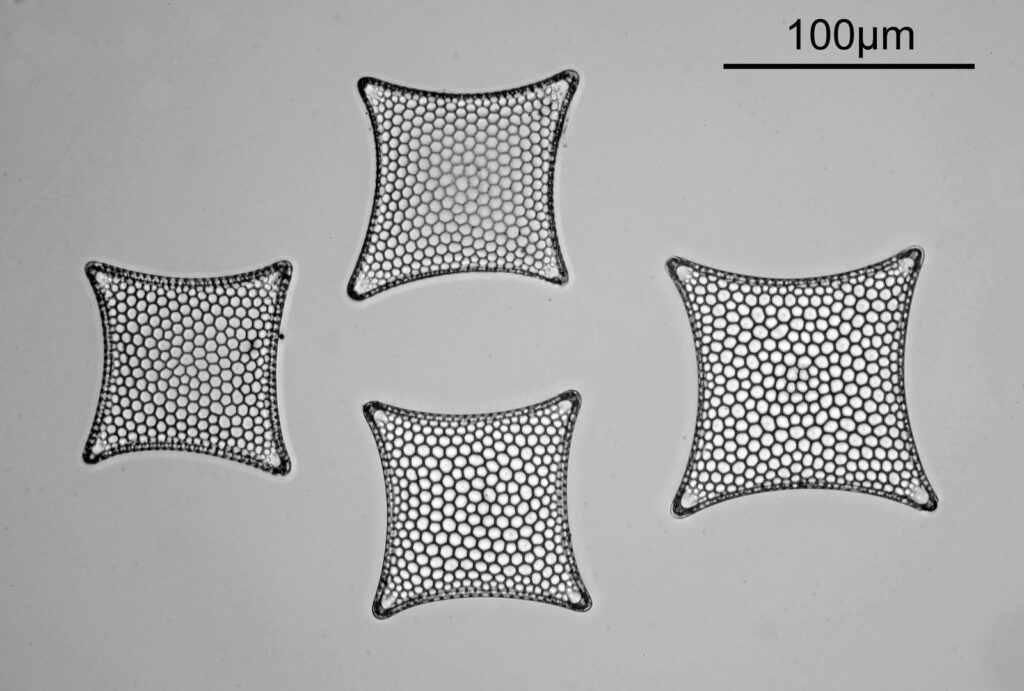
Even closer now with the 20x objective (oblique lighting). There is some overlap between the images.
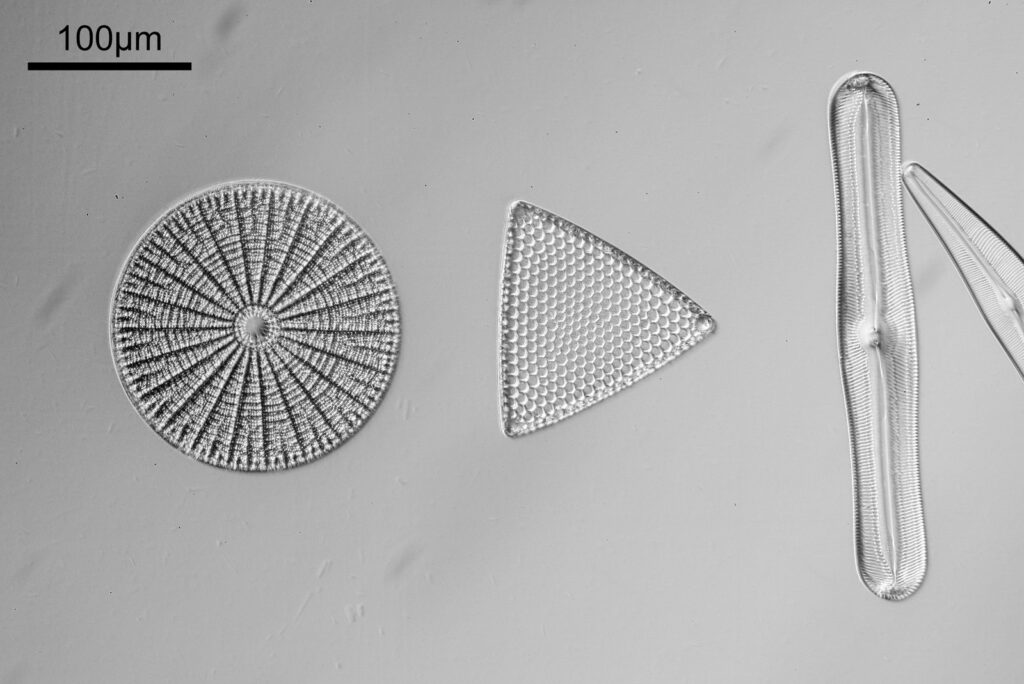
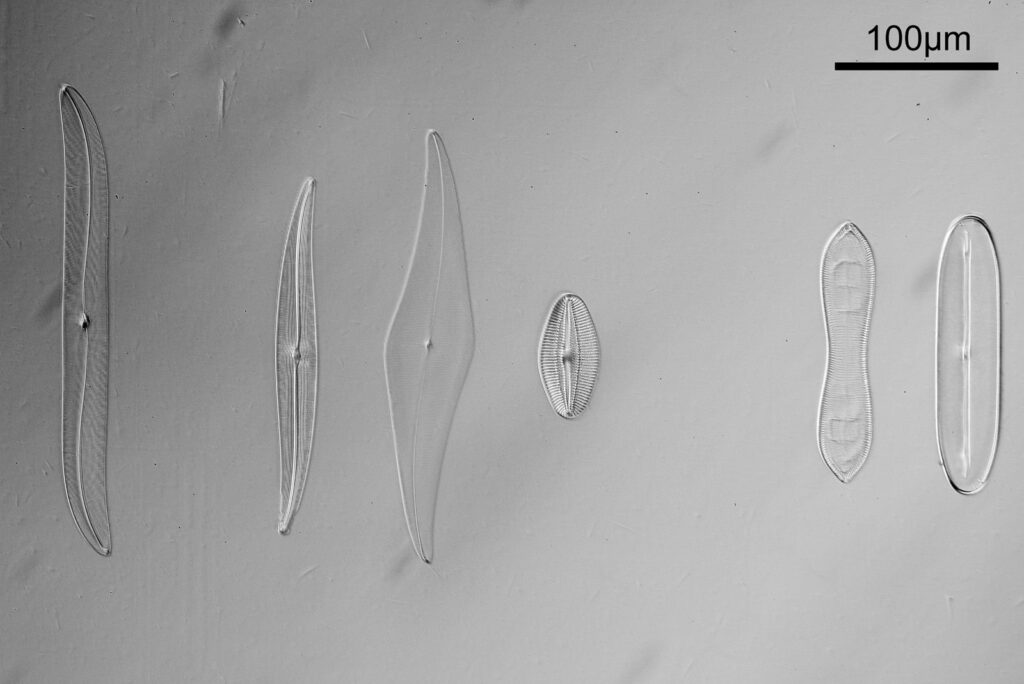
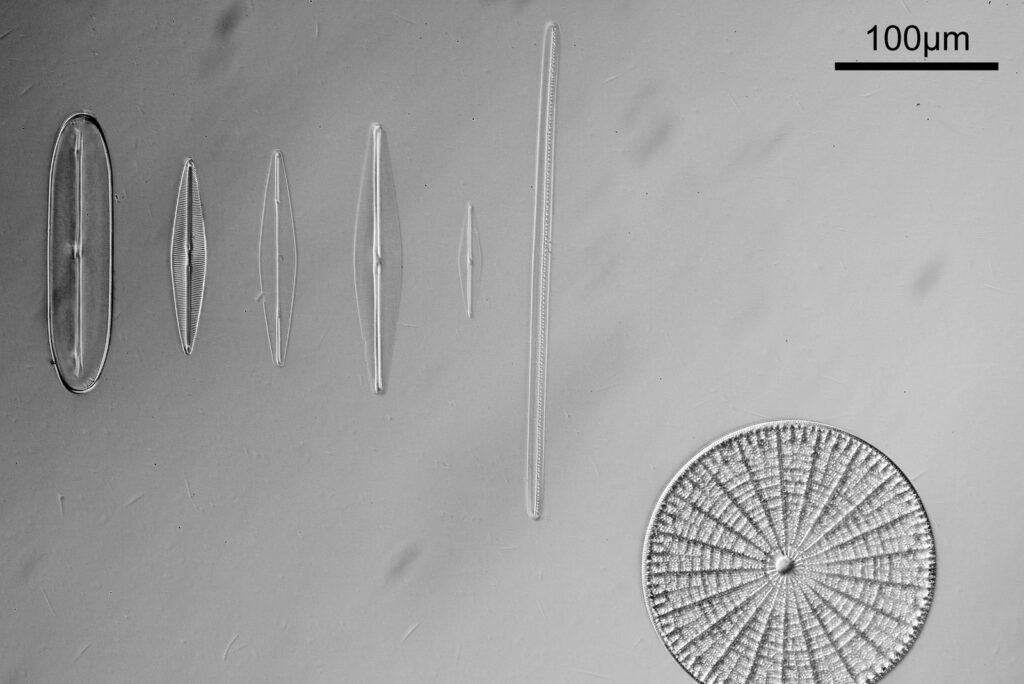
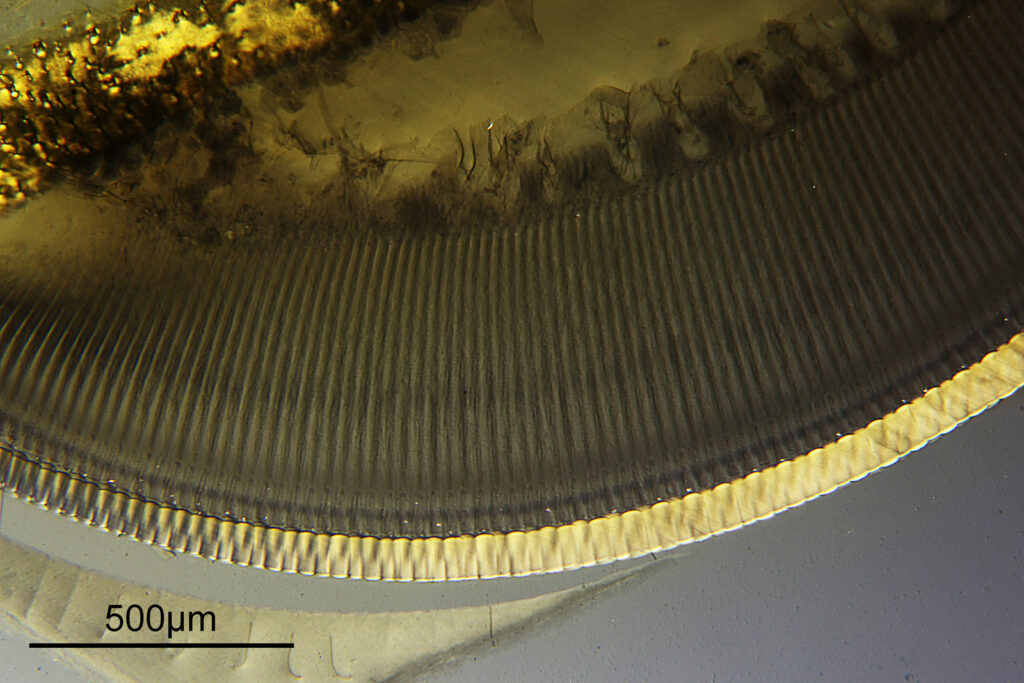
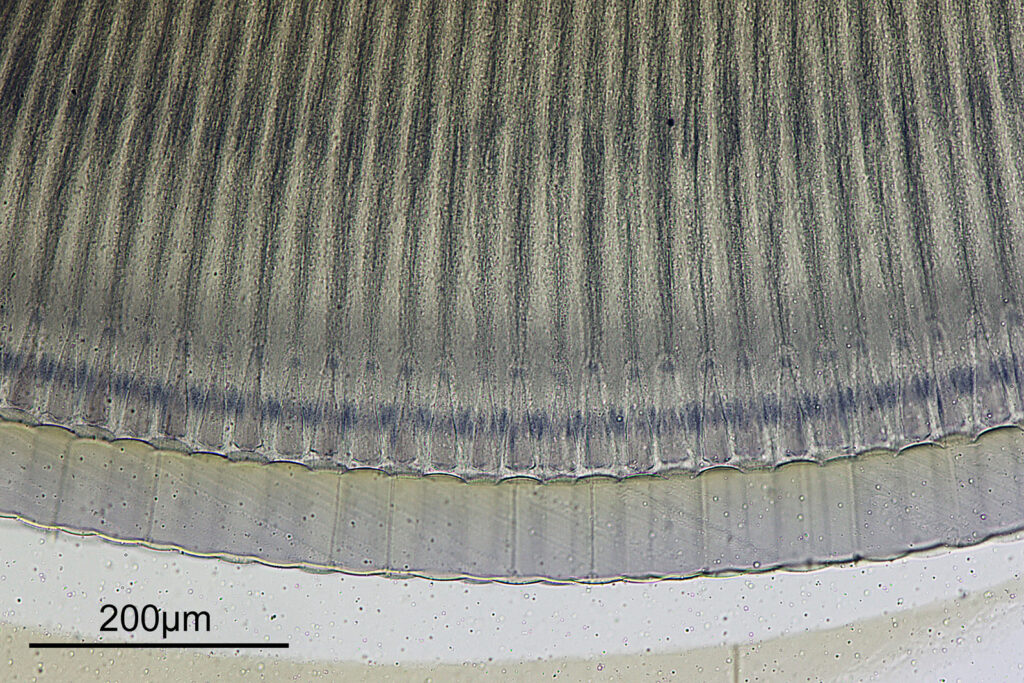
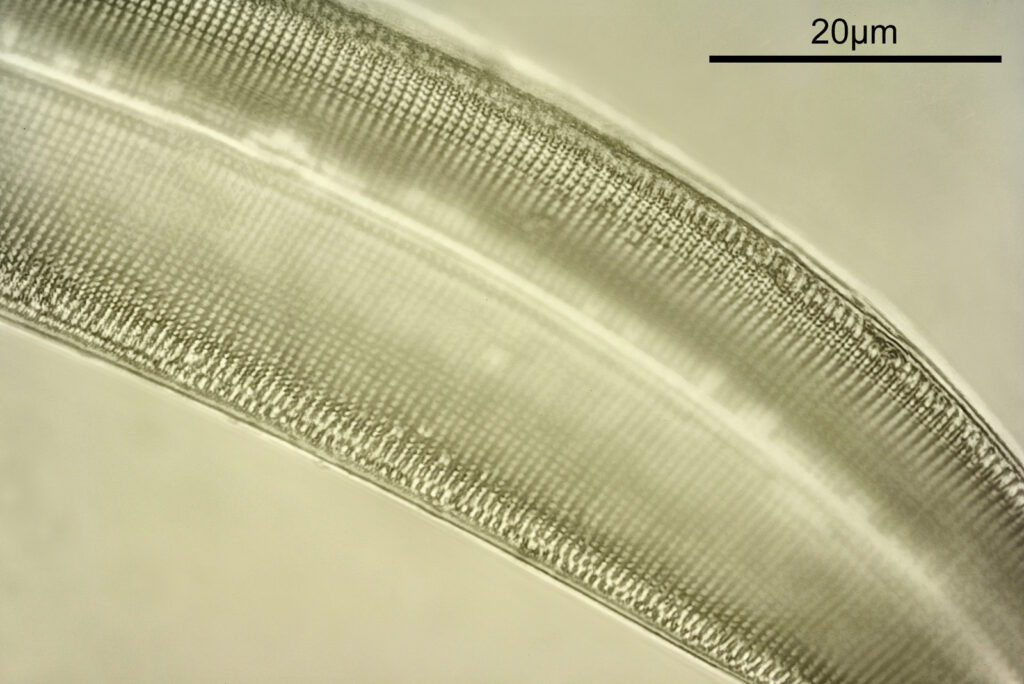
At this magnification, the details were starting to become obvious. So I went in closer to some of the diatoms using a 60x objective (oblique lighting).
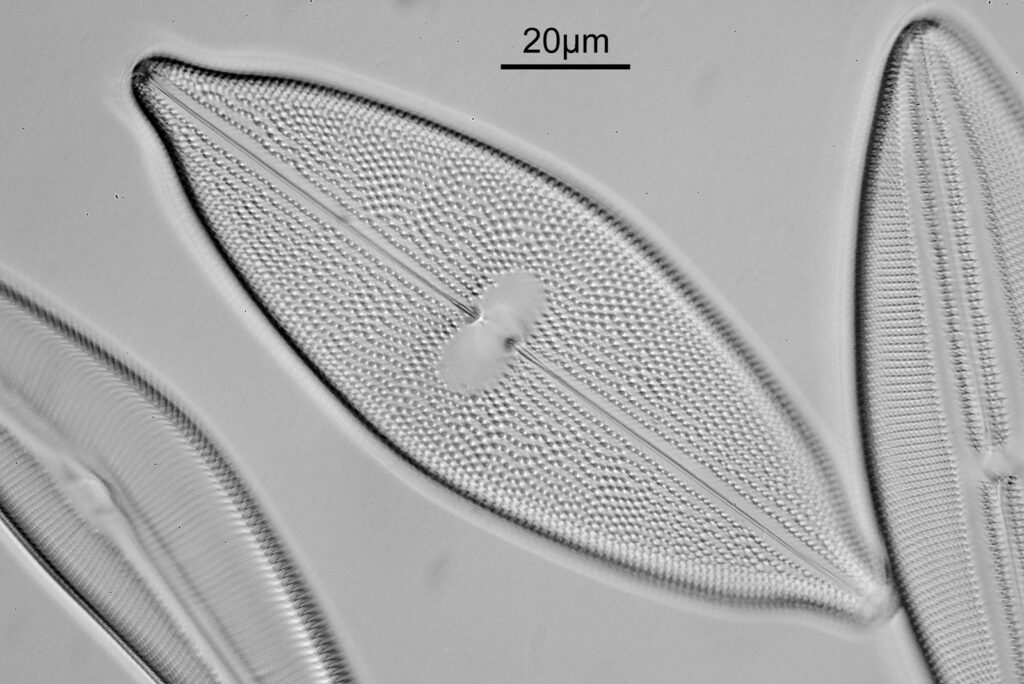
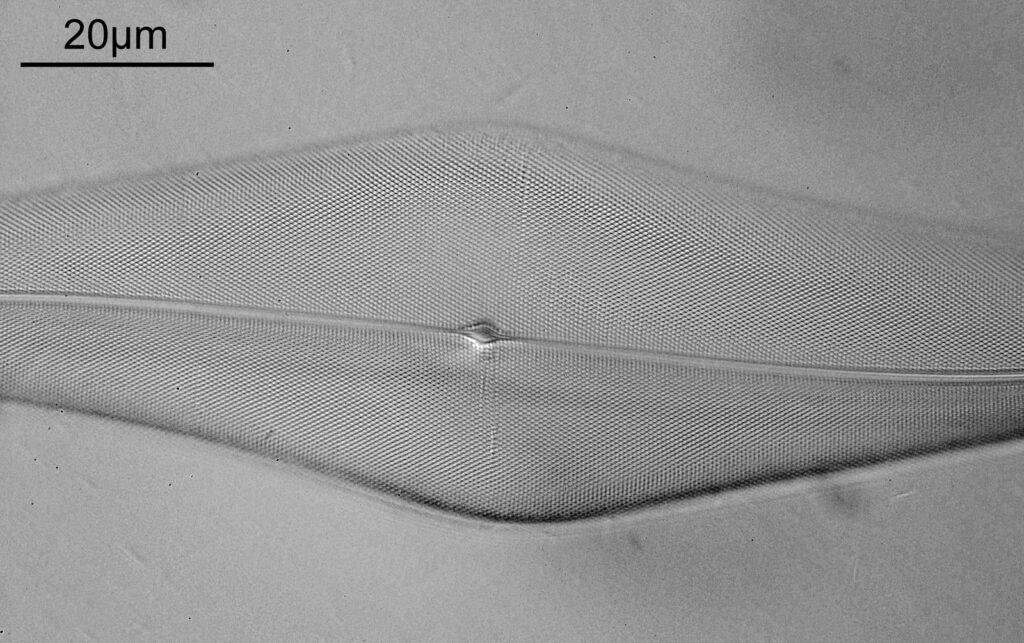
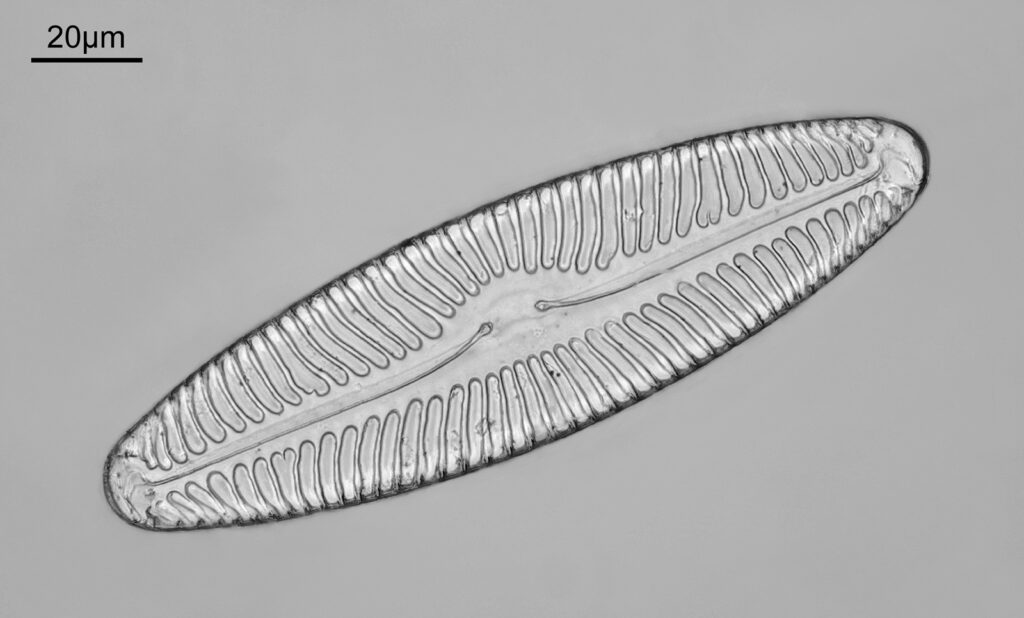
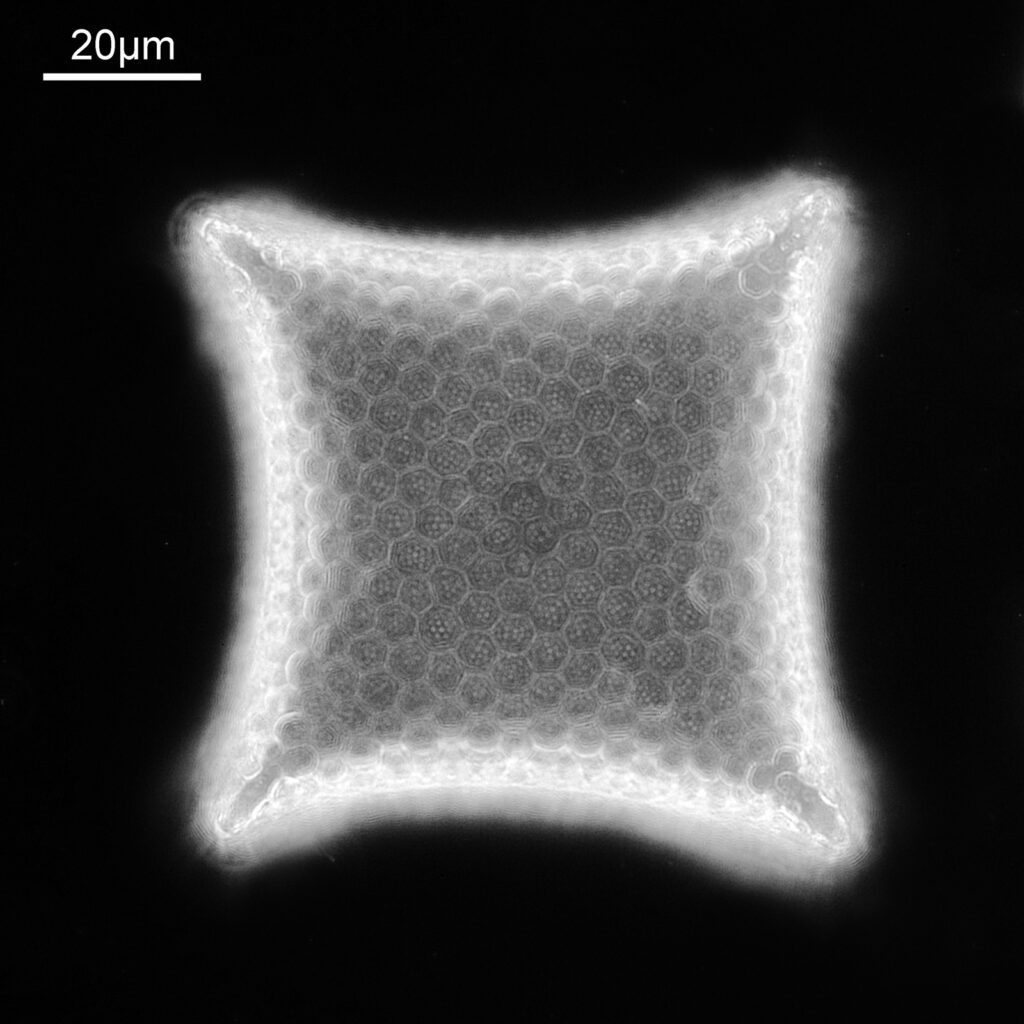
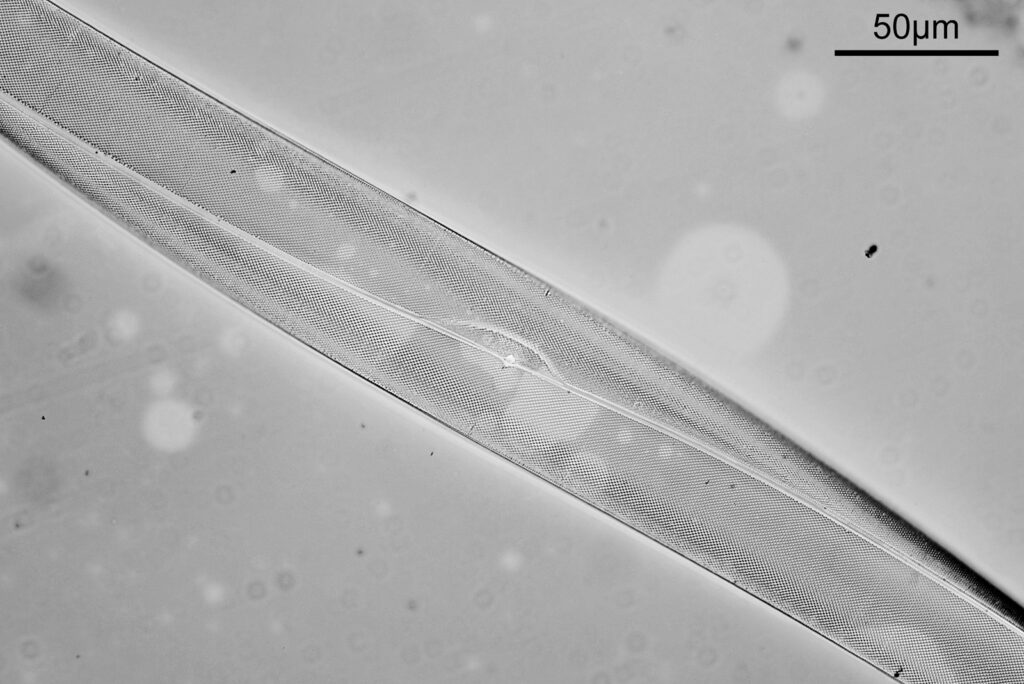
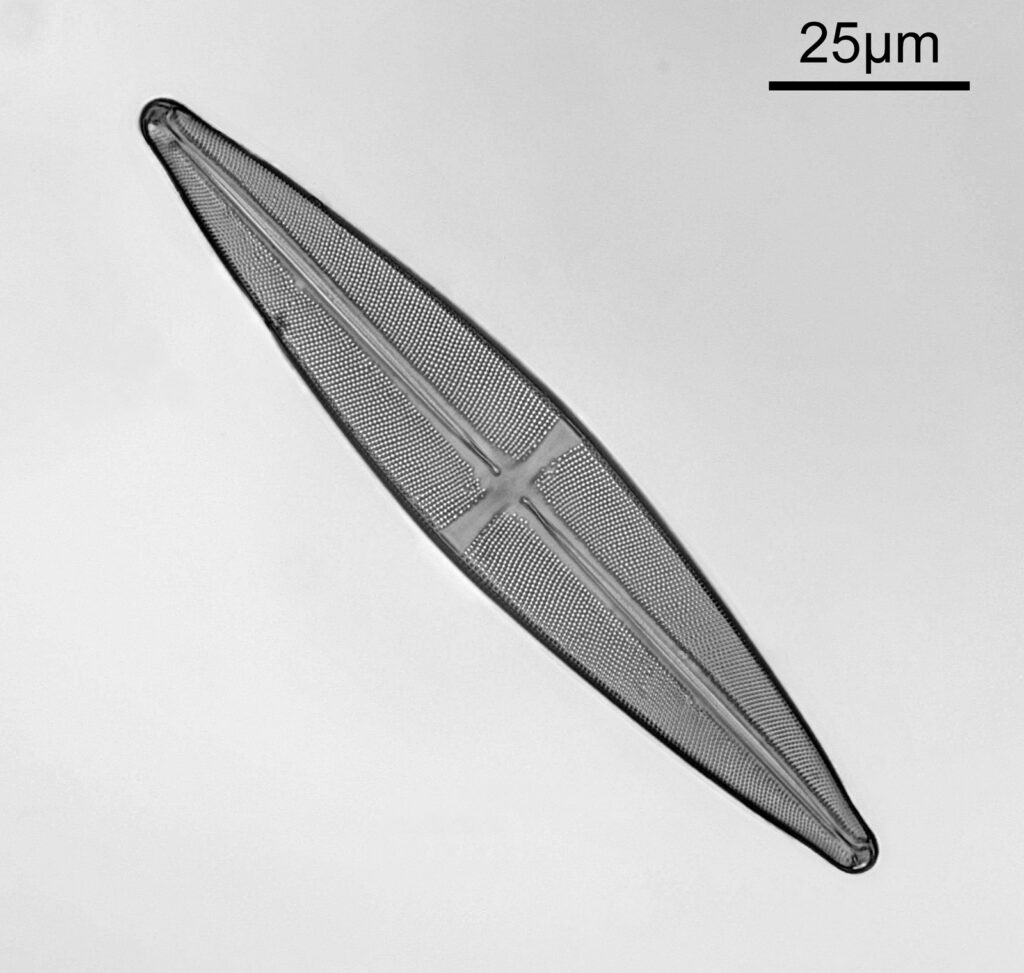
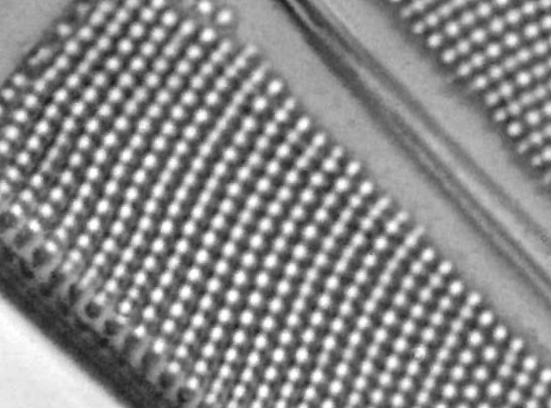
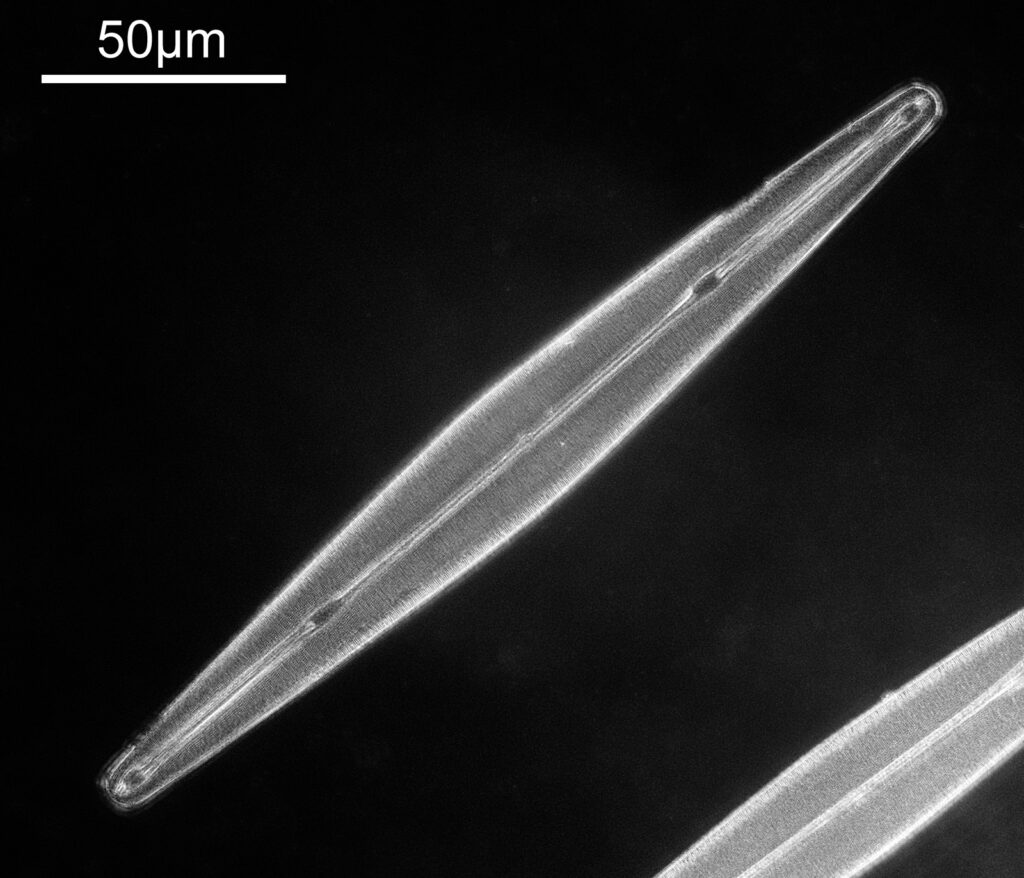
One in particular struck me as very interesting, and it was one which I had struggled with for the name – 13. Navicula lewisiana. I’d not seen one of these before, and here is how it looked with the 60x objective (oblique lighting).

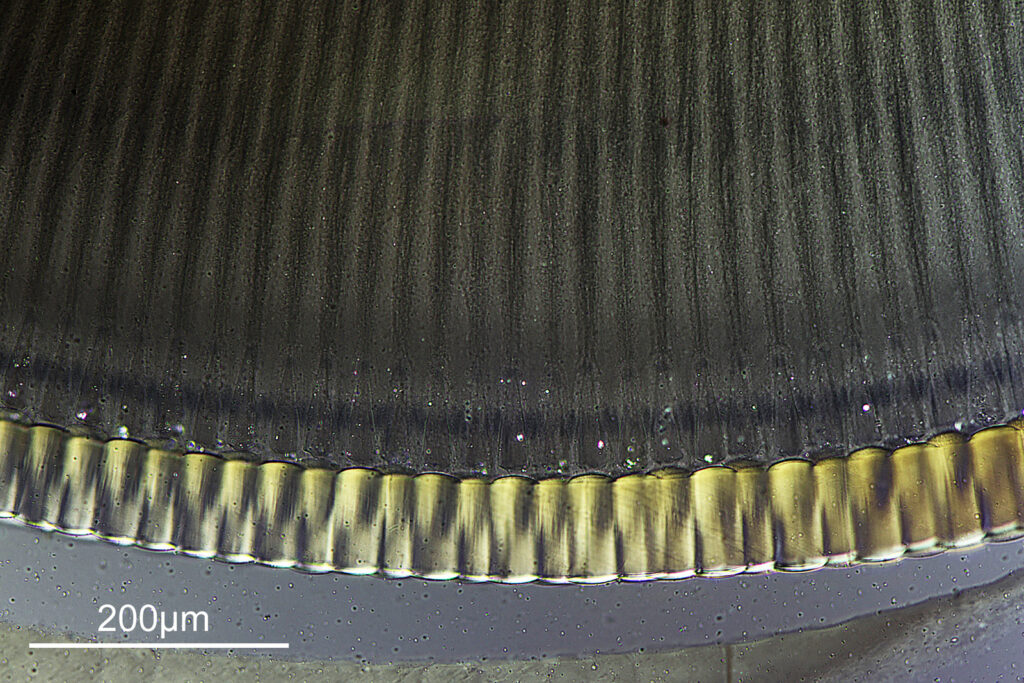
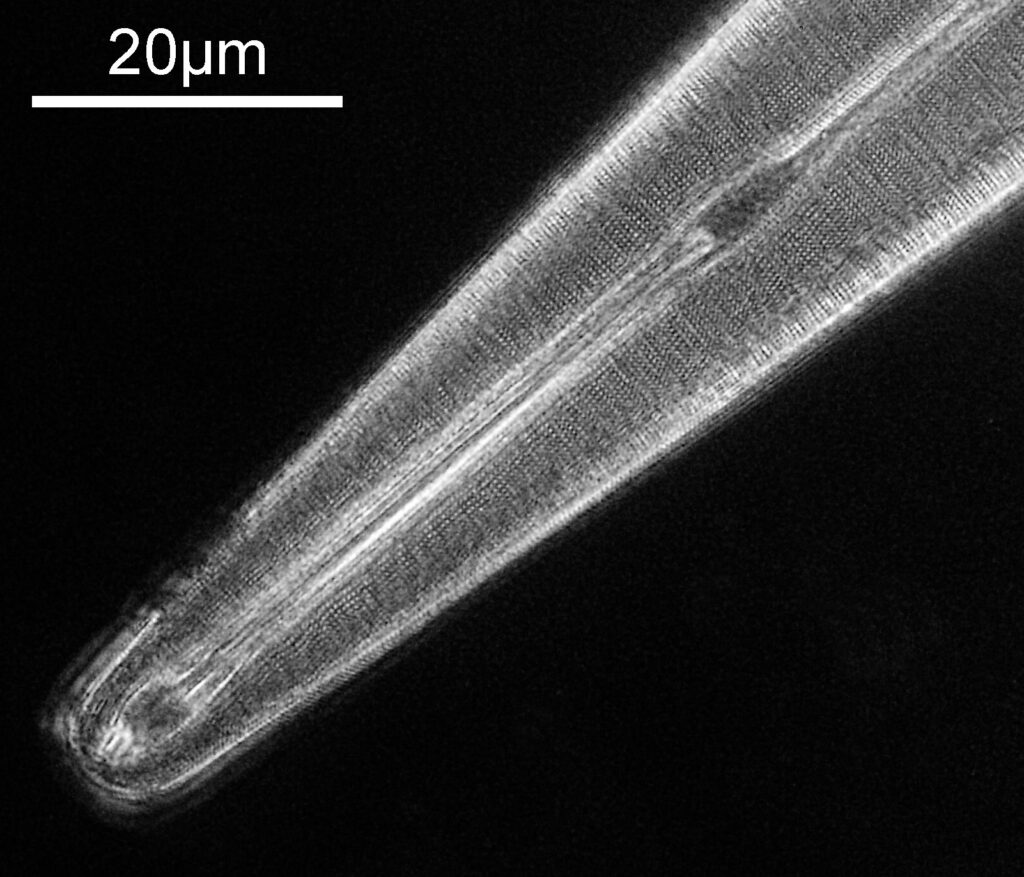
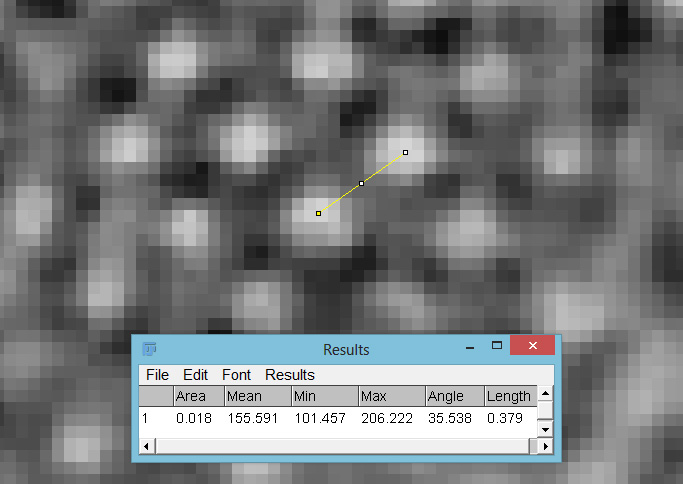
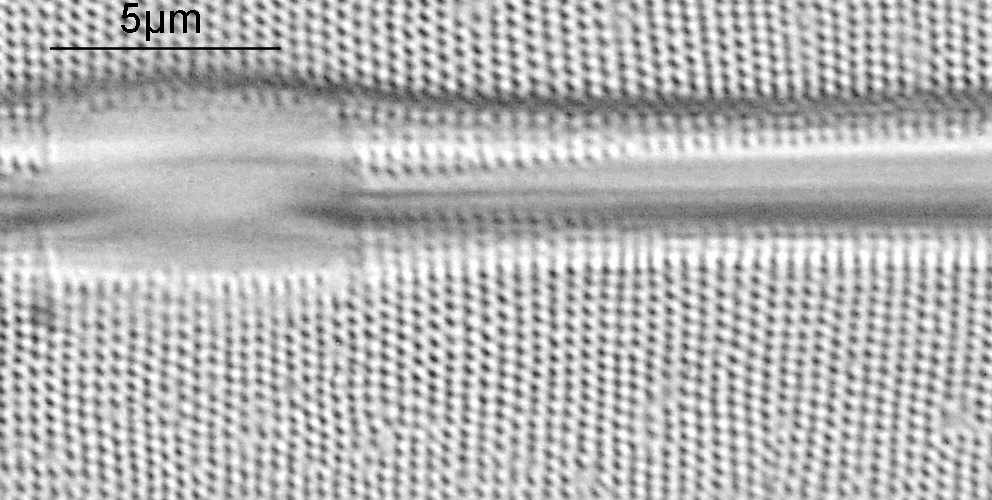
At this resolution the fine detail is lost. Below is a crop from the original. shown at original pixel resolution.
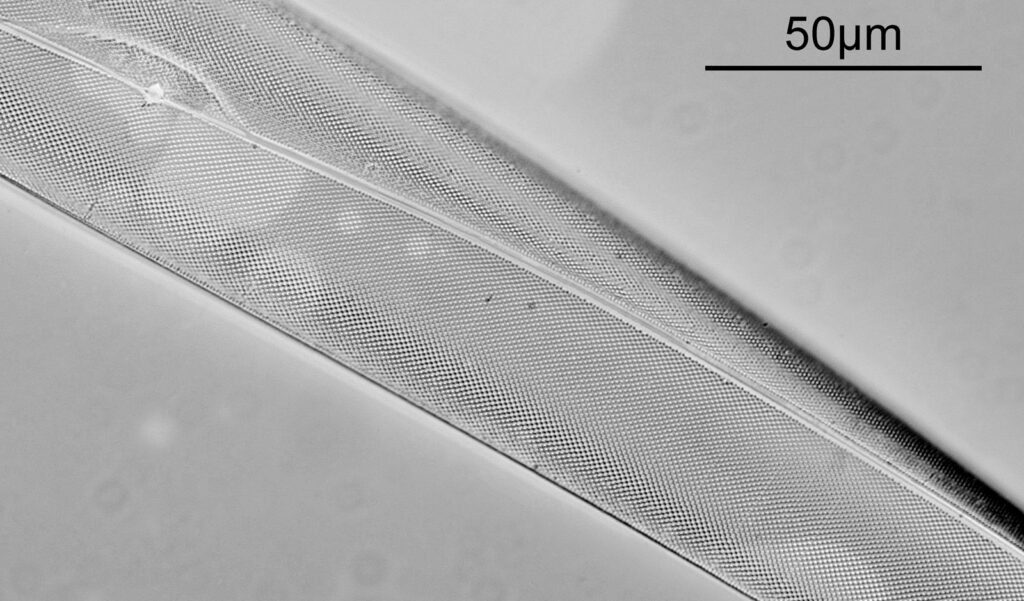
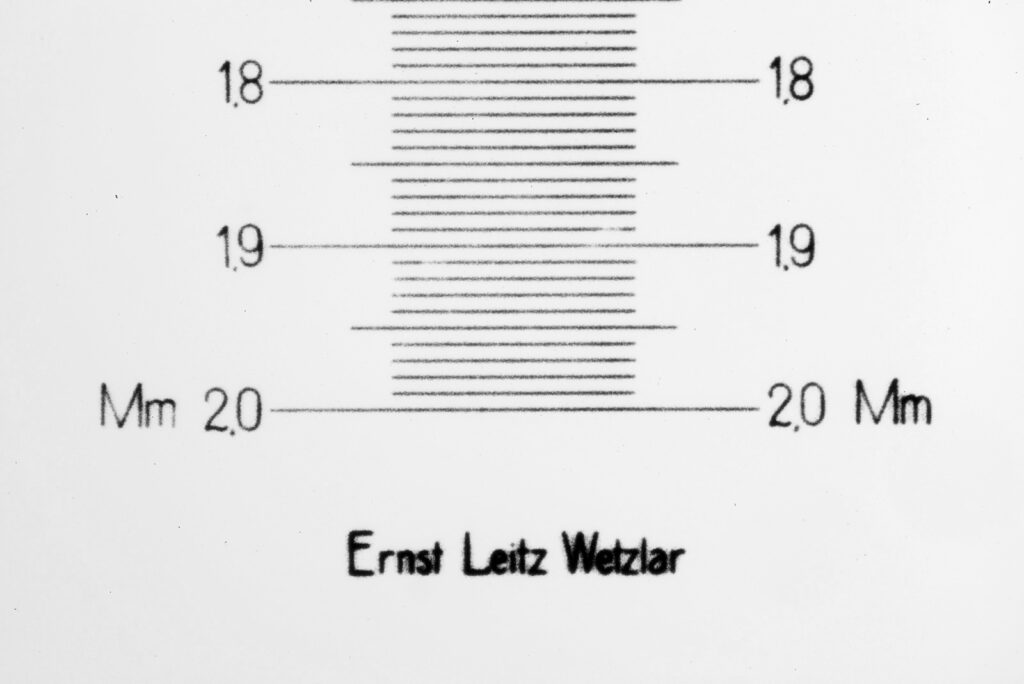
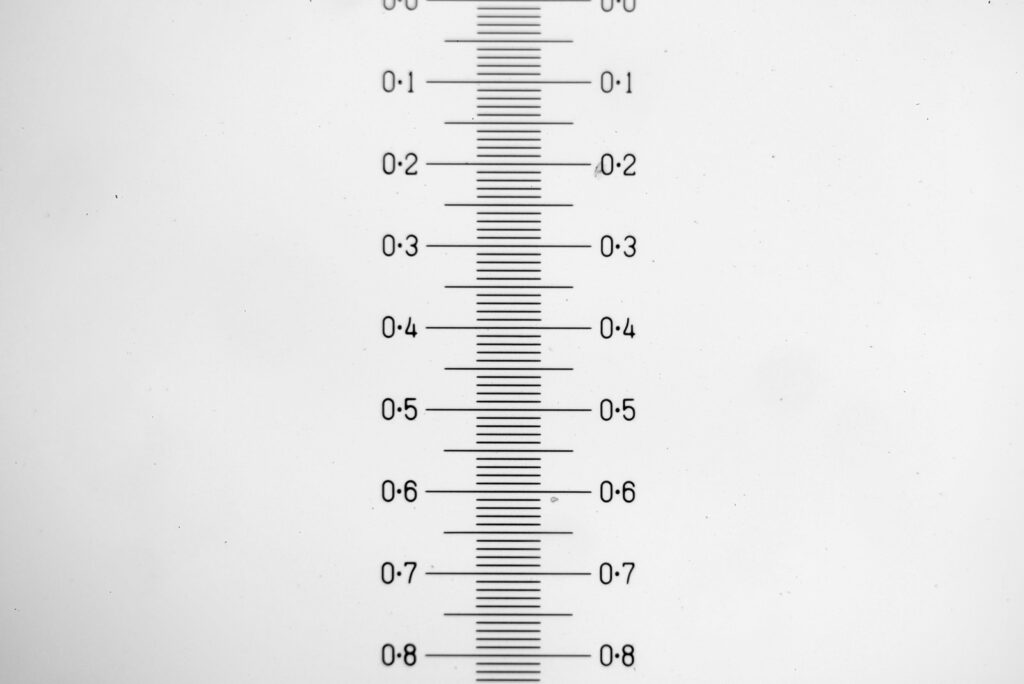
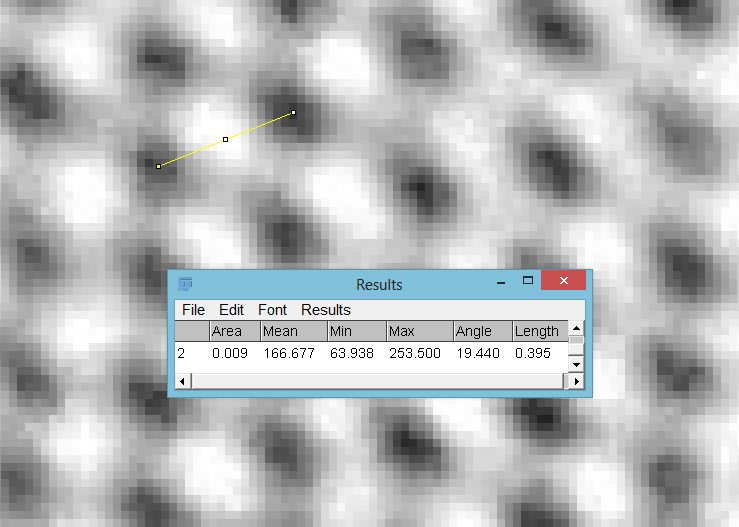
Putting this in ImageJ, the spacing between the poroids can measured, and it comes out to just under 400nm (shown as 0.395 in the image below).
As I mentioned, this was one I hadn’t seen before, but is one I shall come back to again to do more imaging on. Here’s some information on it on the Diatoms.org website. Also, it is in the excellent book “The diatoms”, by Round, Crawford and Mann, page 534, where is it mentioned that it is “A rarely recorded monotypic genus.”. Explains why I haven’t seen it before. While I am on the topic of books, I should also note that there is a bit of information about Meakin in “Microscopical mounts and mounters” by Bracegirdle (published by the Quekett Microscopical Club) which is a text I often refer to for getting background on mounters and slide makers.
As always, thanks for reading, and if you’d like to know more about my work, I can be reached here.
EDIT – I did return to the slide and did some more imaging of the Navicula lewisiana. Same objective (60x Splan Apo) but this time with 405nm light and stacking. In order to get a successful stack I reduced the extent to which the light was oblique, so this is essentially a bright field image.

While there is now detail to the edge of the diatom, the image is a bit ‘flat’ – going away from oblique light and stacking makes it look less 3D to me. This one was tidied up more as well. I actually think I prefer the 450nm light image which wasn’t stacked, but this is more of a complete image of the diatom. Horses for courses as they say……