Today’s post has come about as the result of a chat with a microscope shop who I use to check over my objectives when they need fixing (JB Microscopes Ltd in Newcastle). A few weeks ago I bought an 20x Olympus Splan Apo that had some optical issues (the price was right and I had hoped it was worth the gamble). To see if it could be saved I sent it to JB Microscopes to be looked over and hopefully fixed. Unfortunately it could not, but while I was chatting with them I asked what objectives they had on their shelves. It turned out that they had a couple that I had been looking for for a while – a Nikon 40x UV-F glycerine immersion objective, and a Leitz 63x Pl Apo oil immersion objective. This post will cover some initial results with the Nikon 40x UV-F.
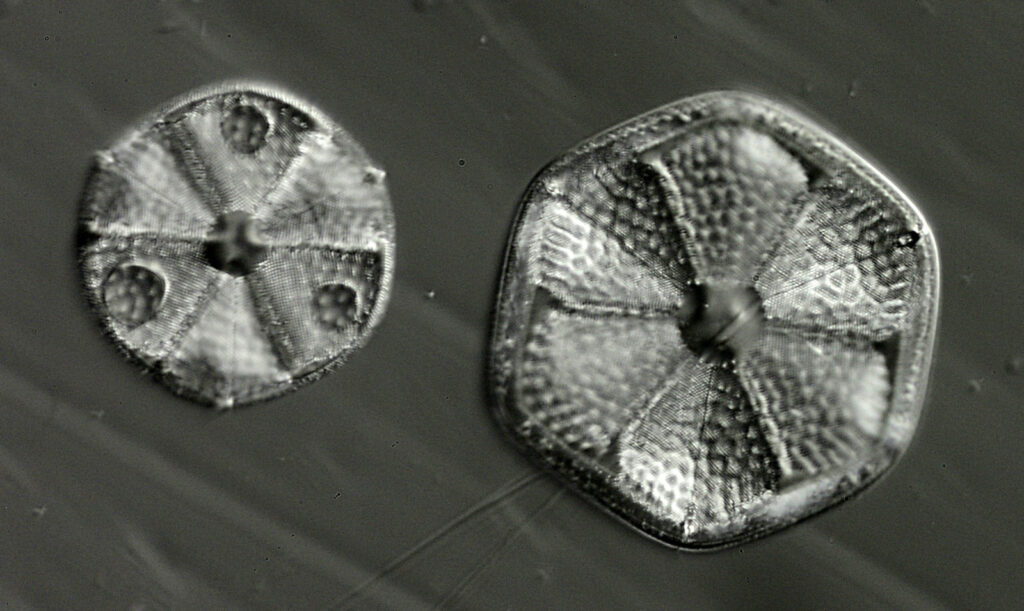
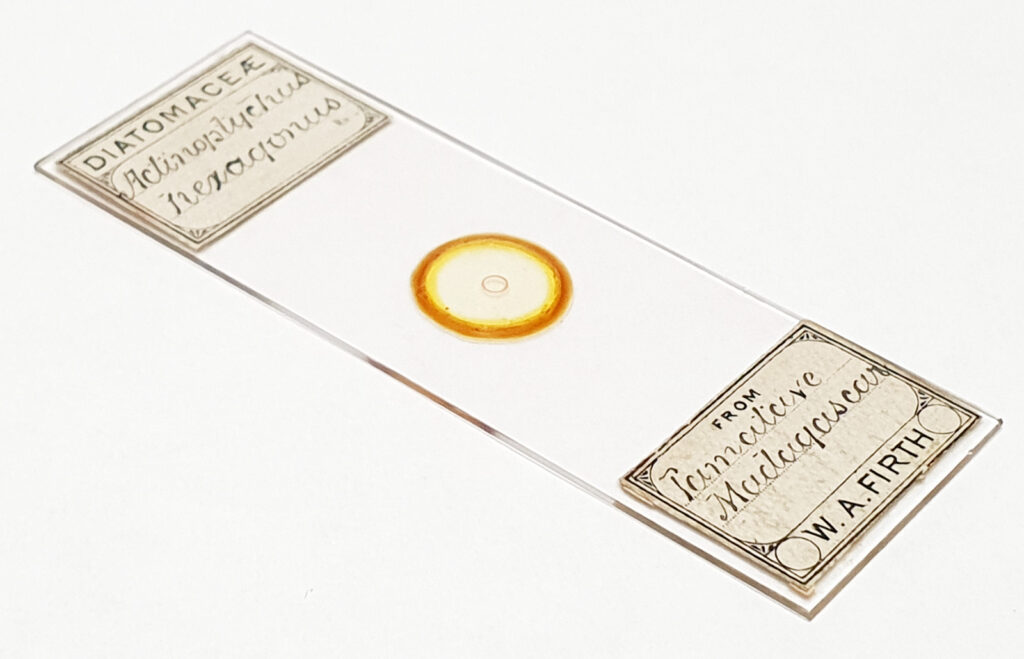
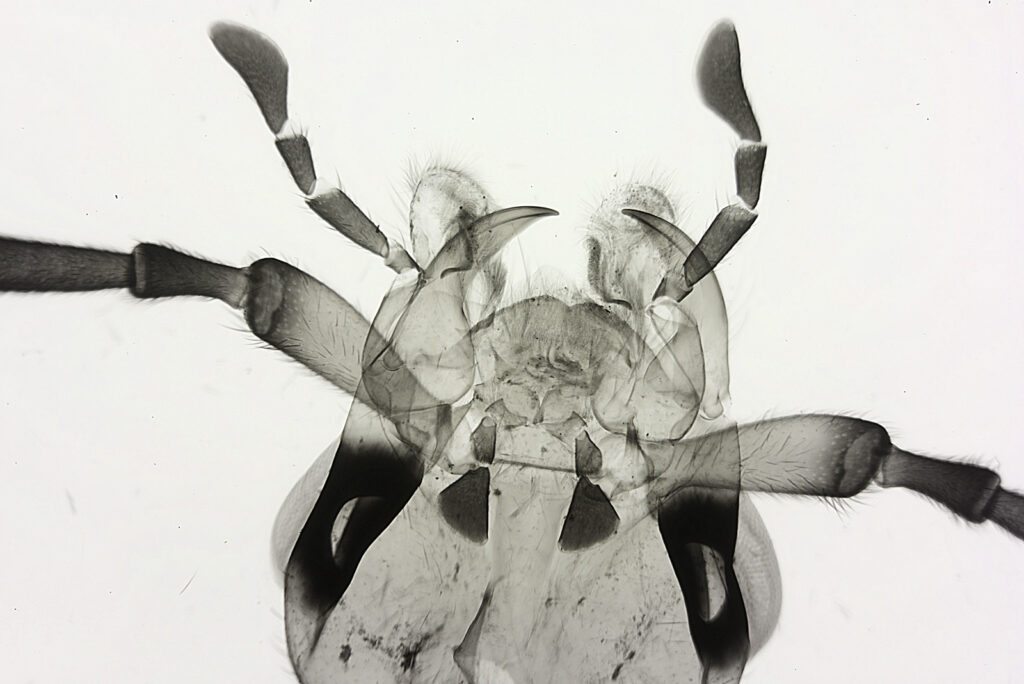
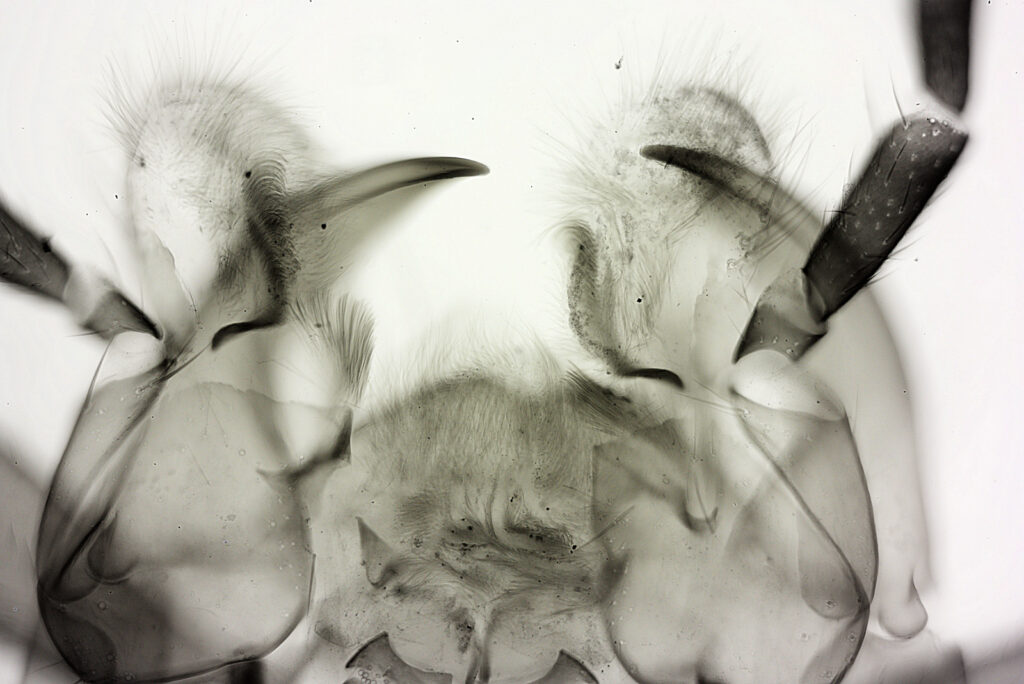
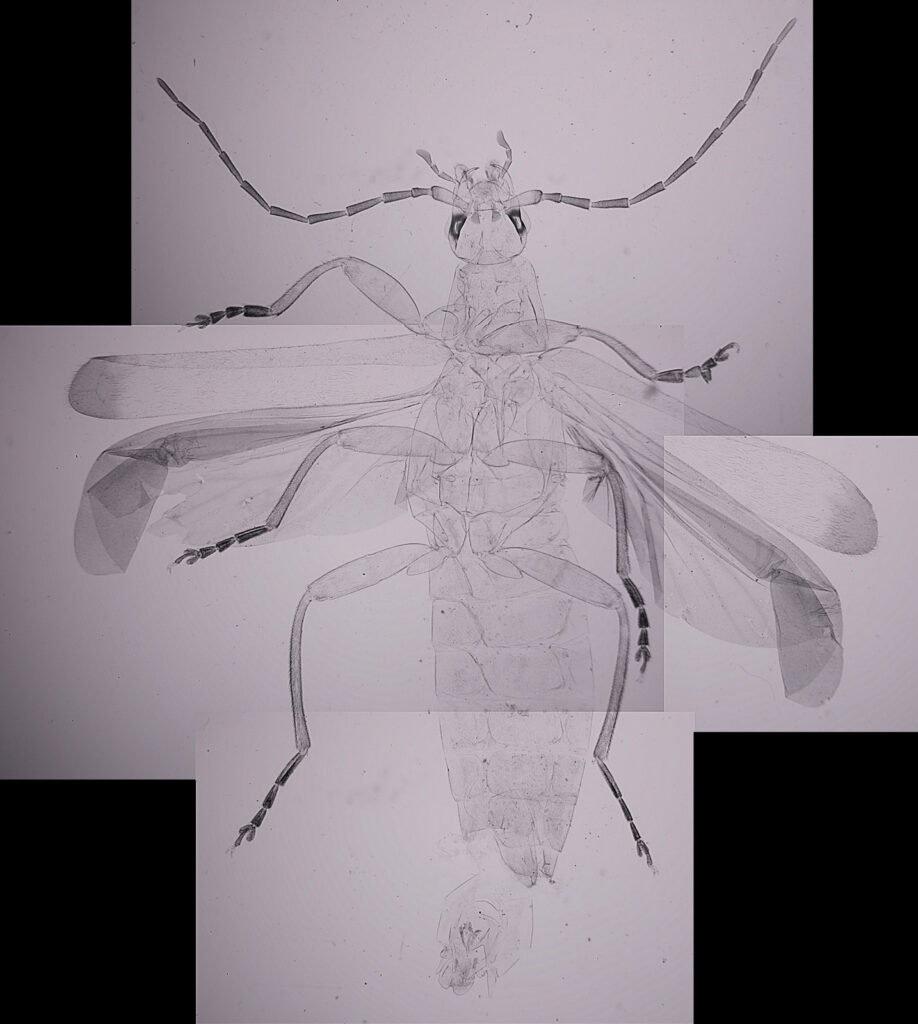
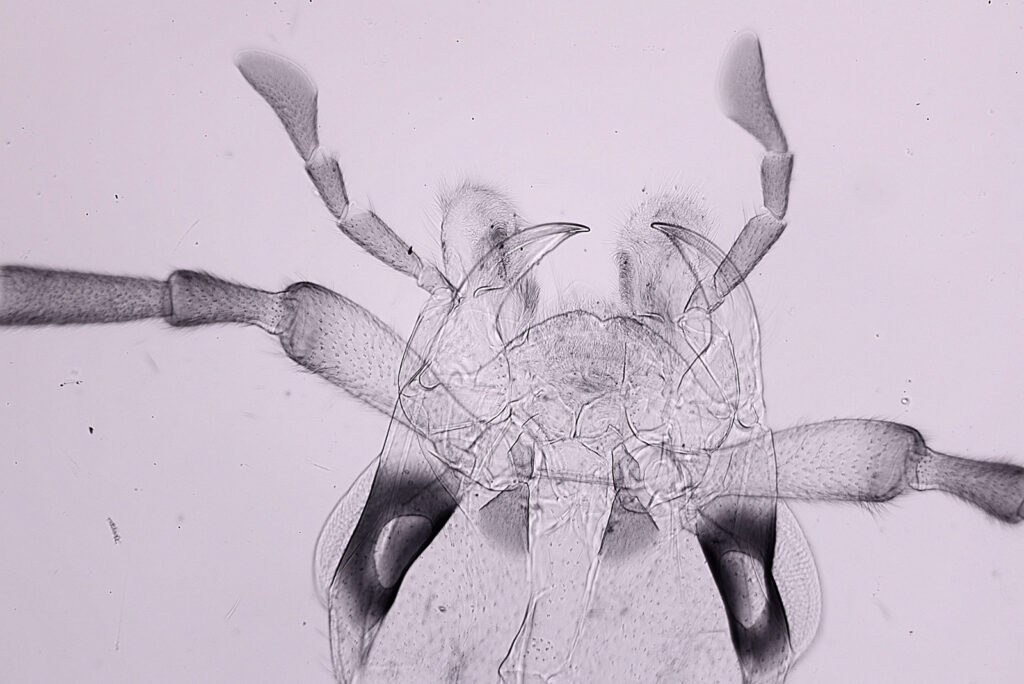
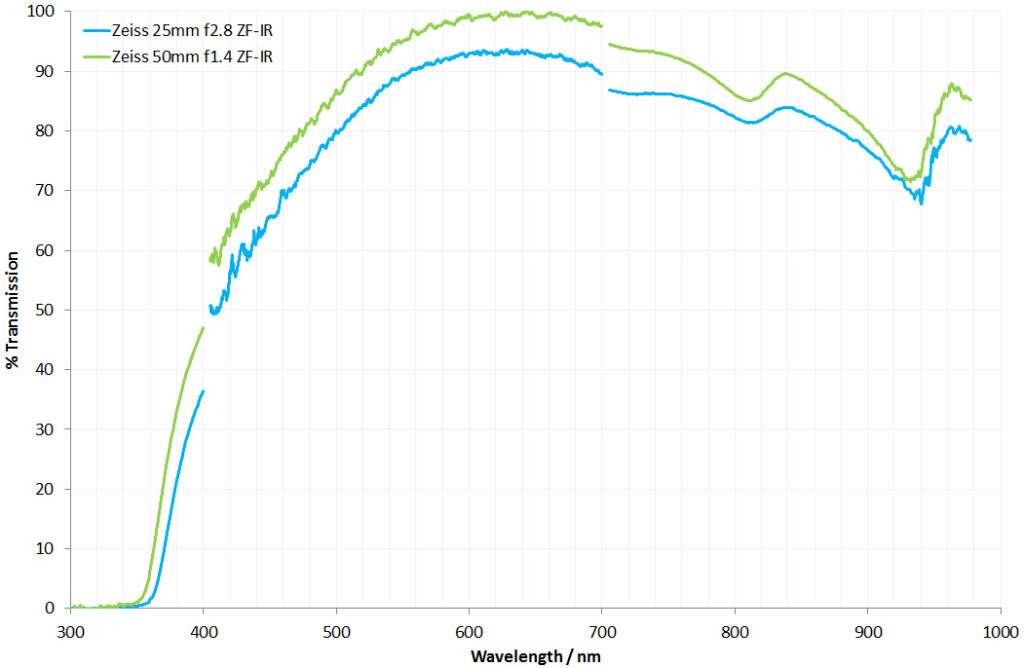
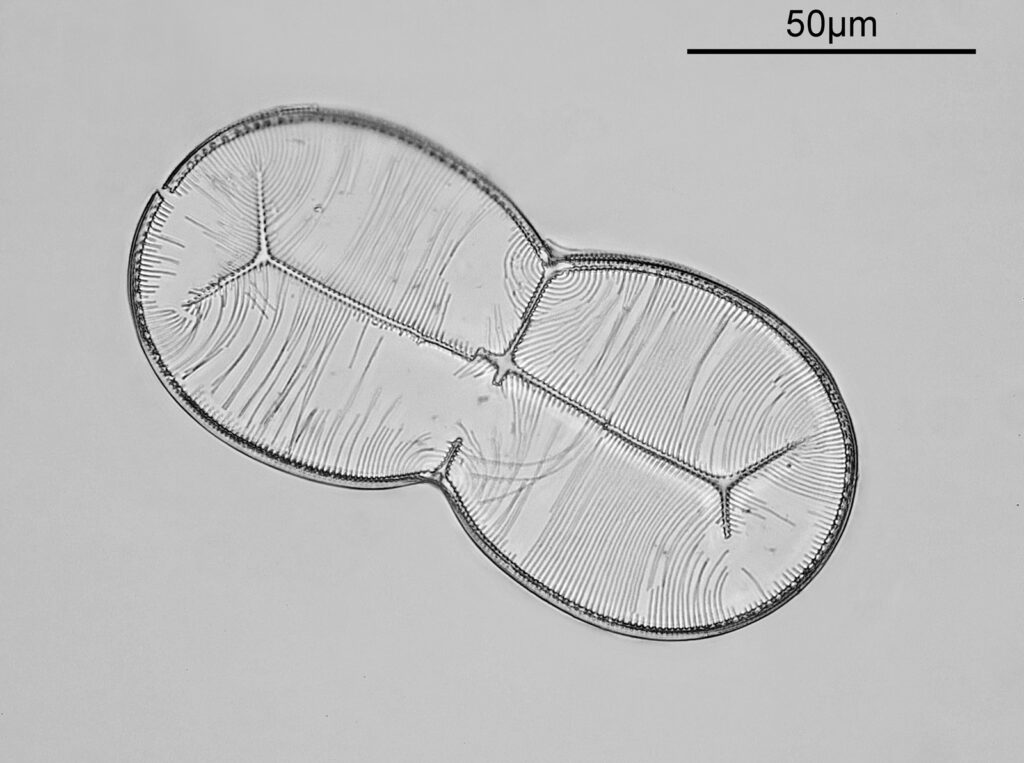
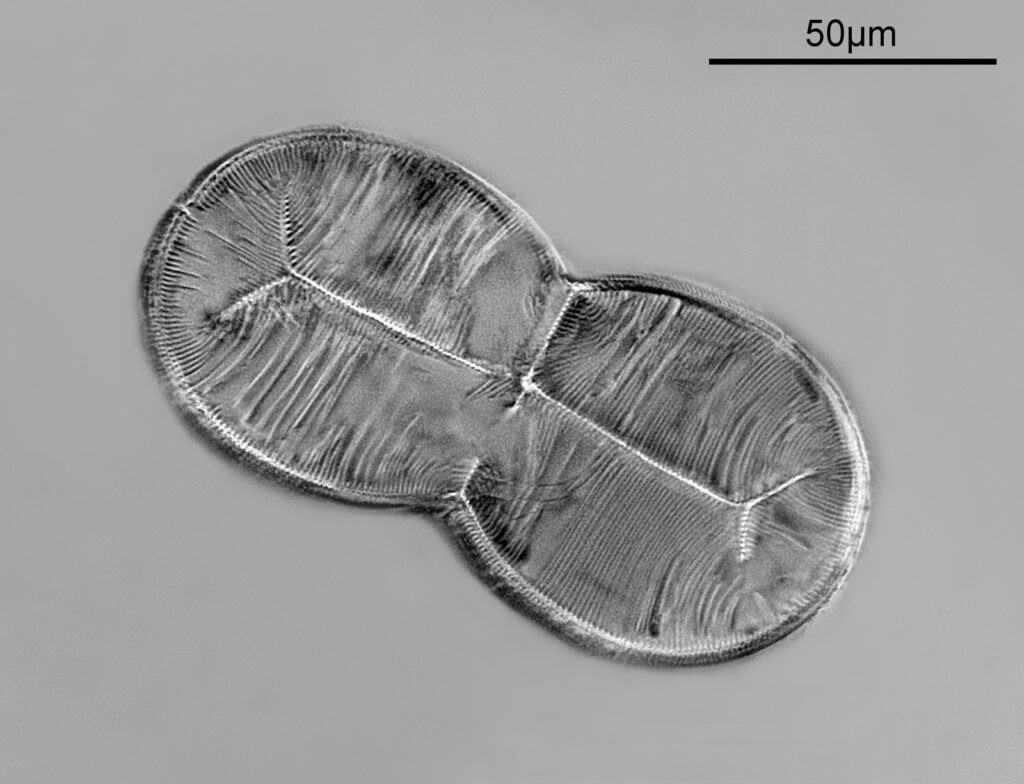
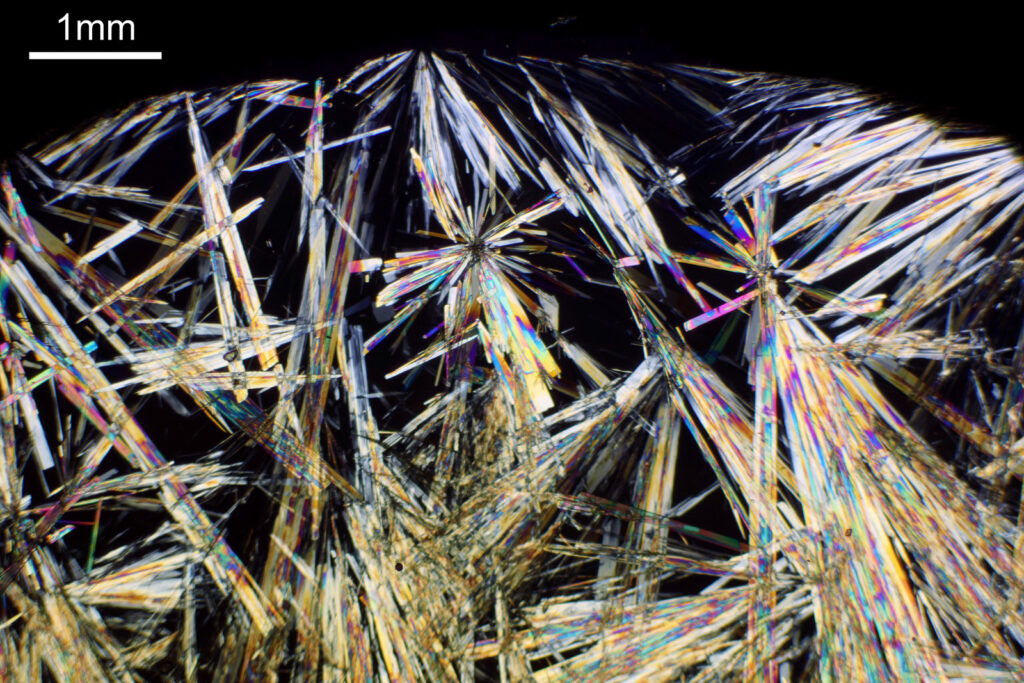
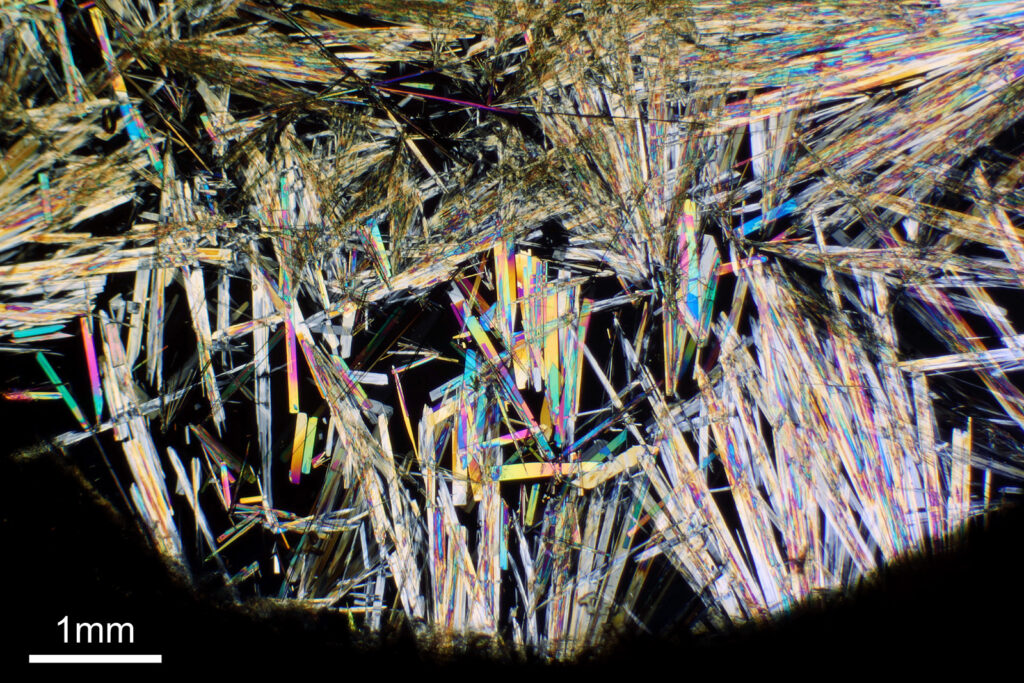
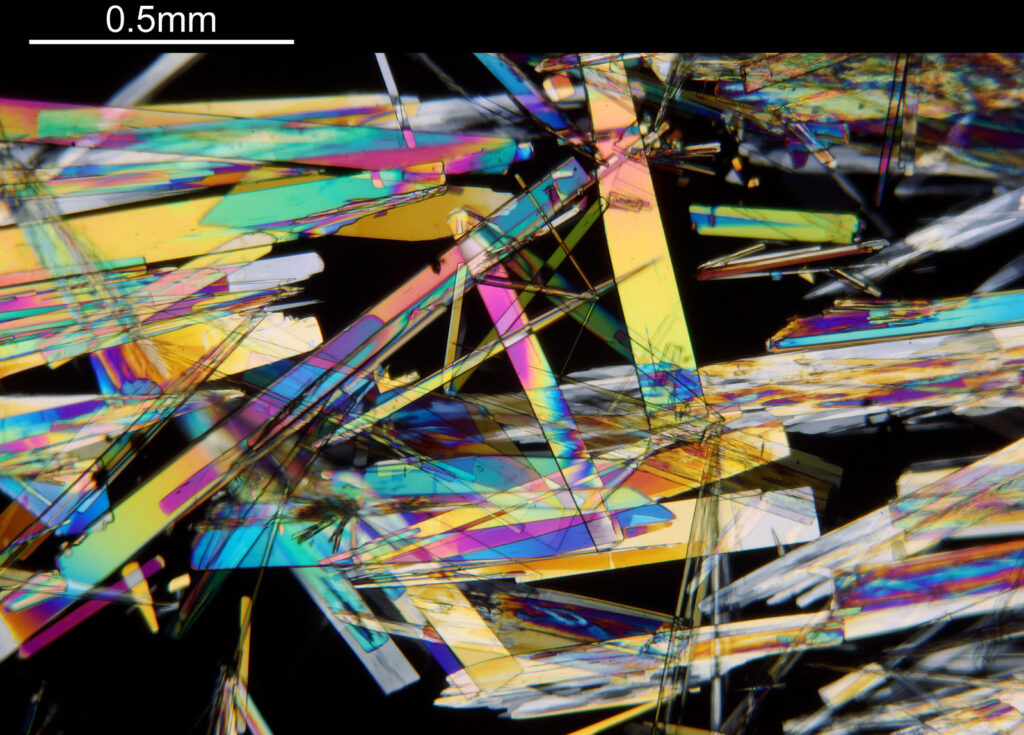
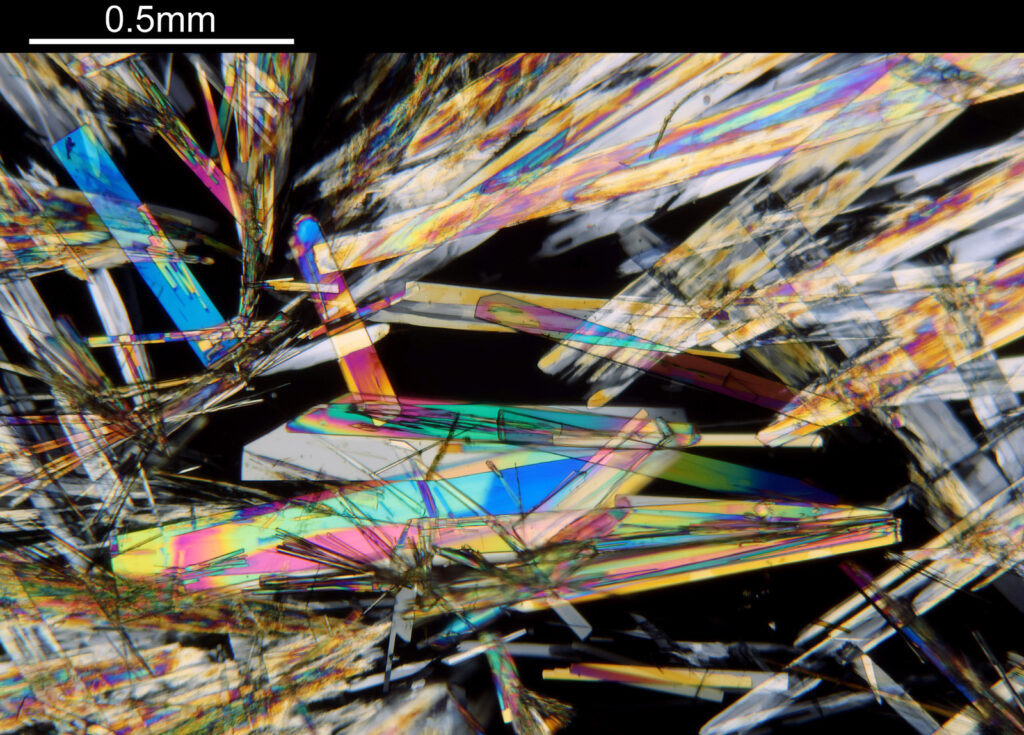
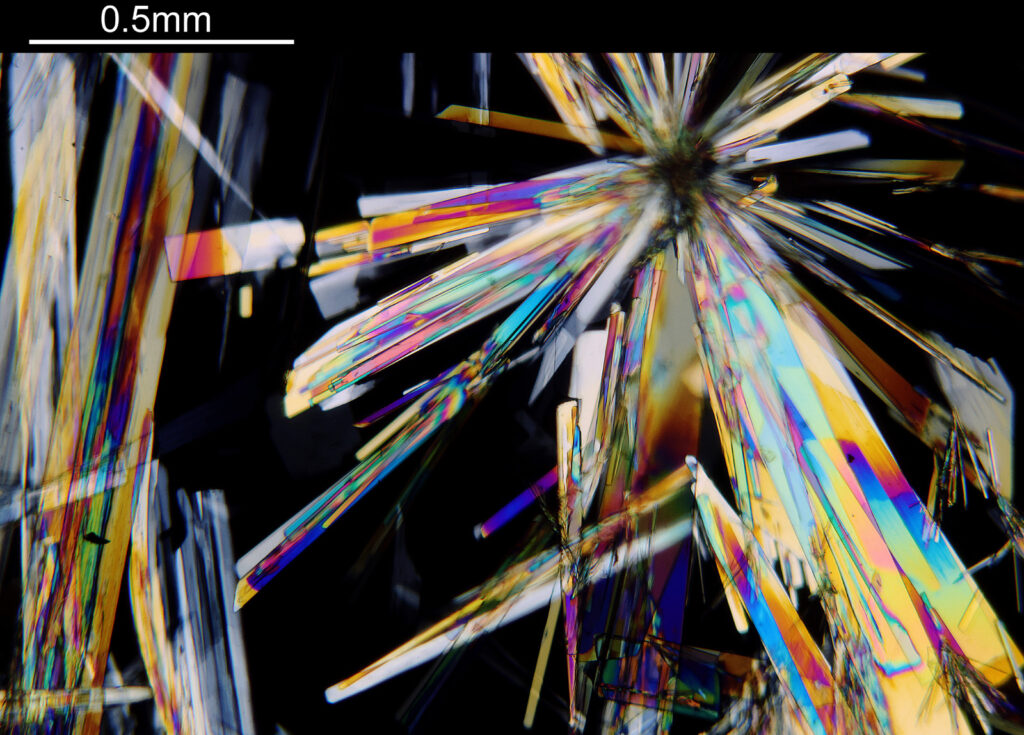
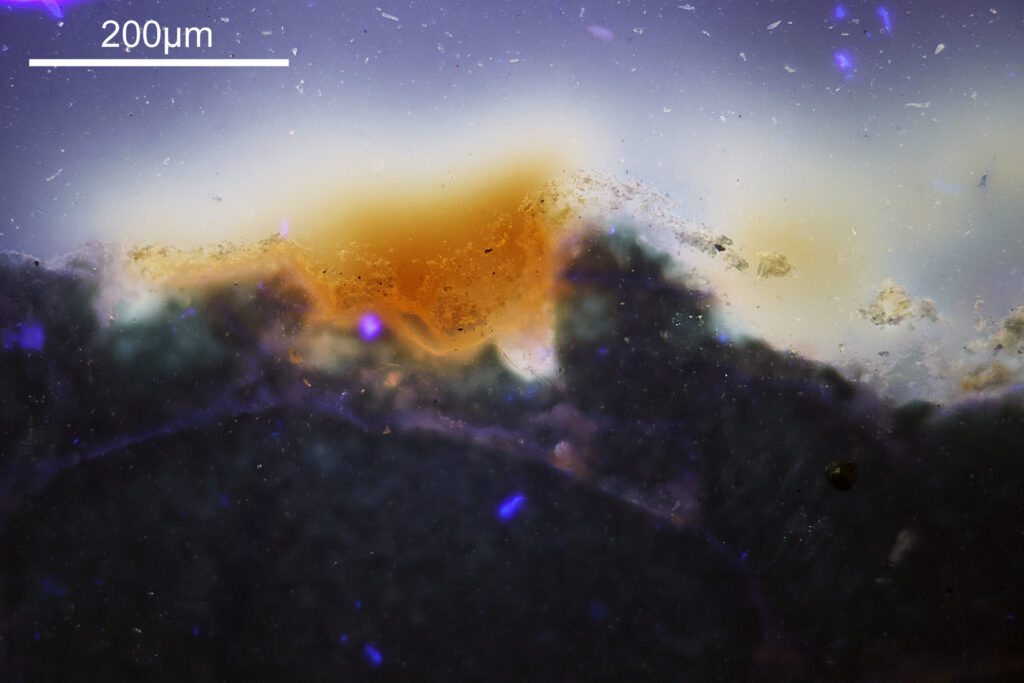
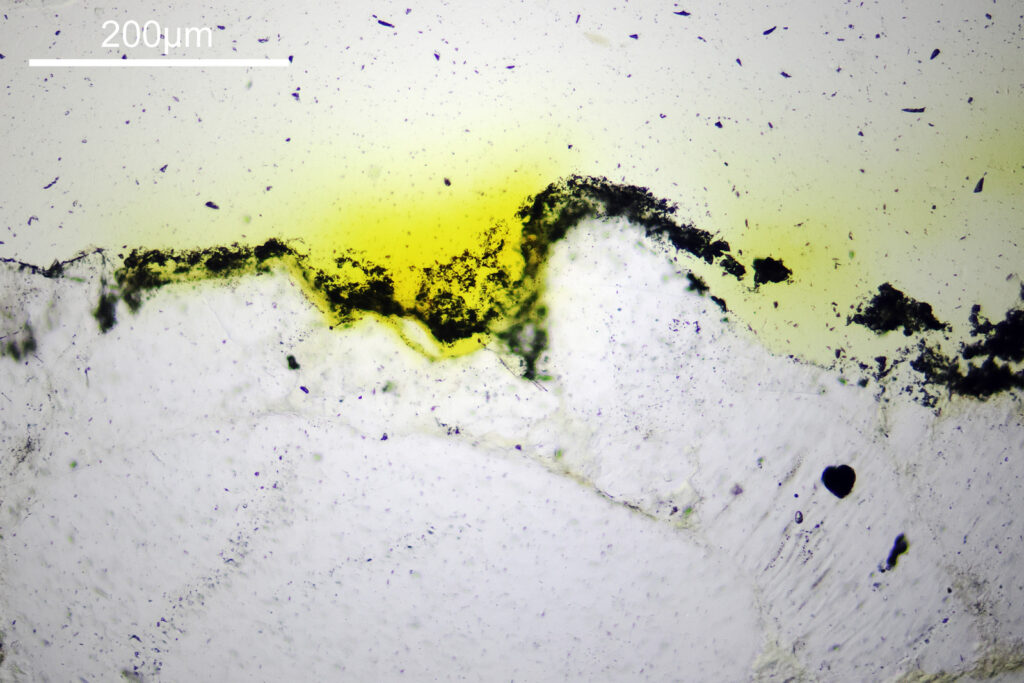
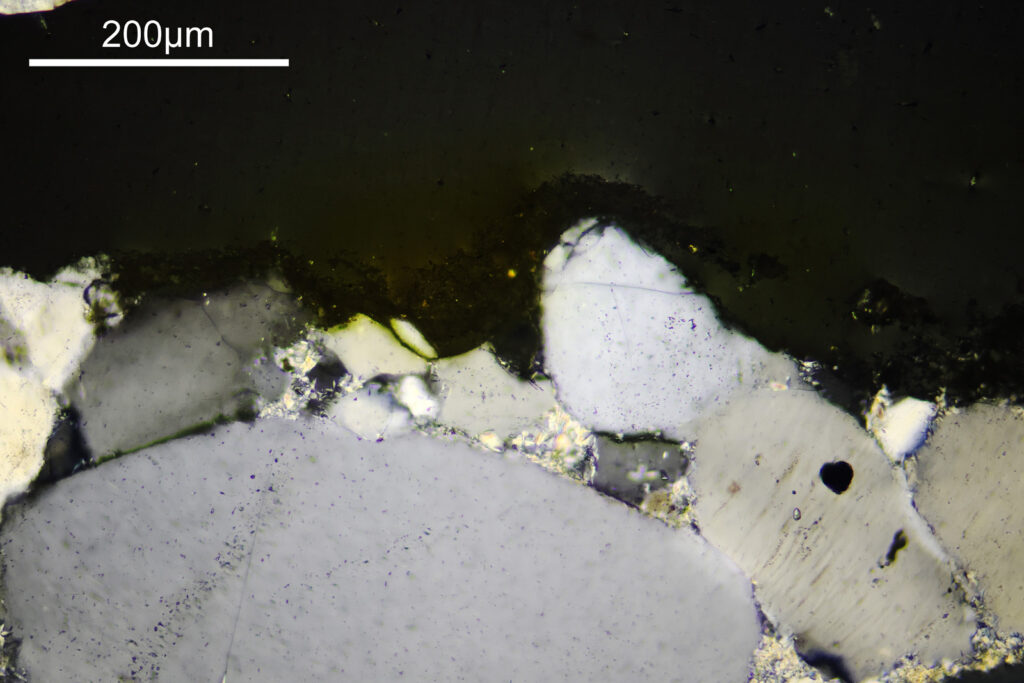
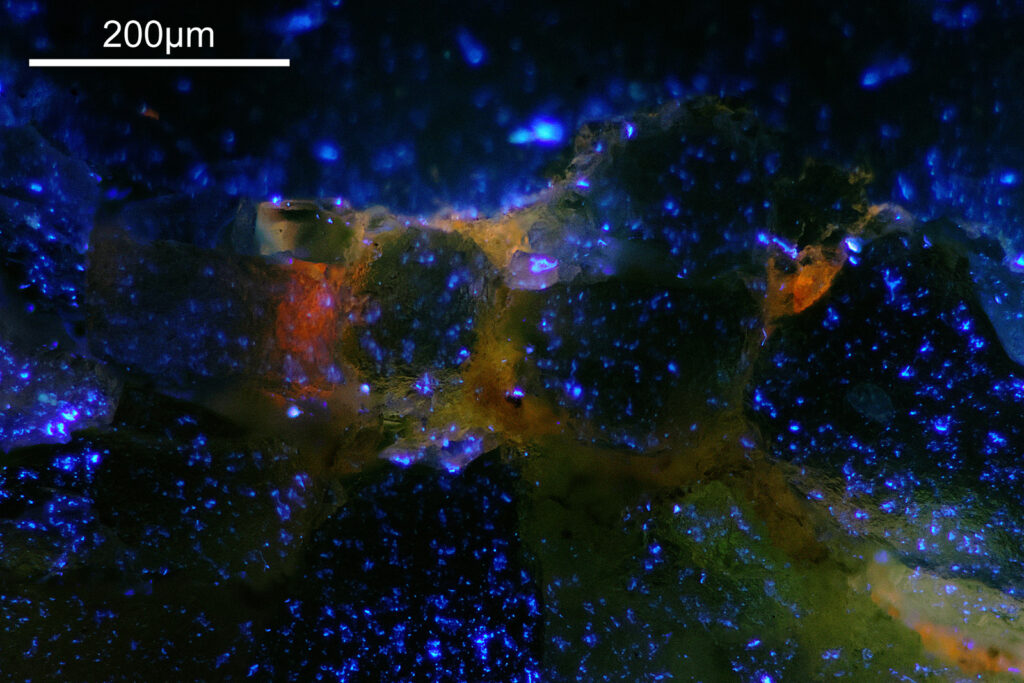
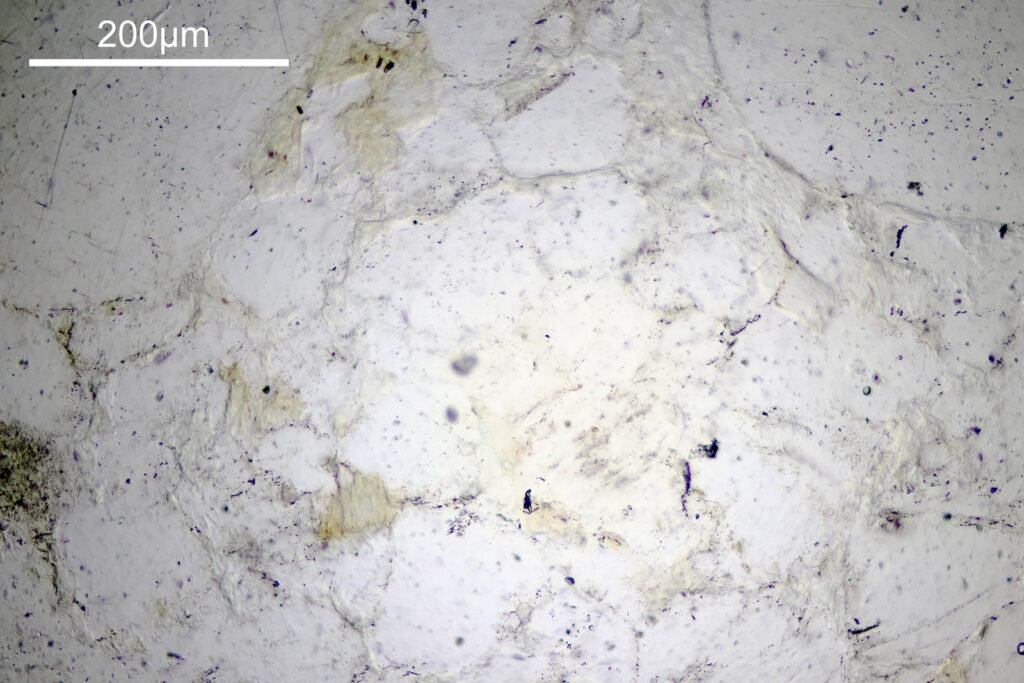
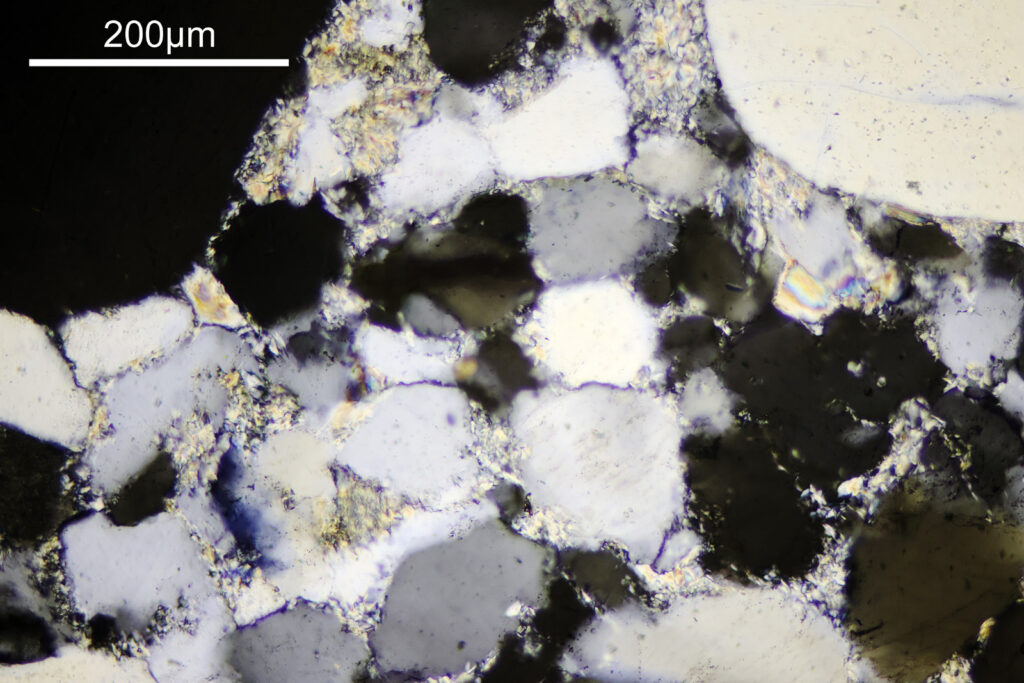
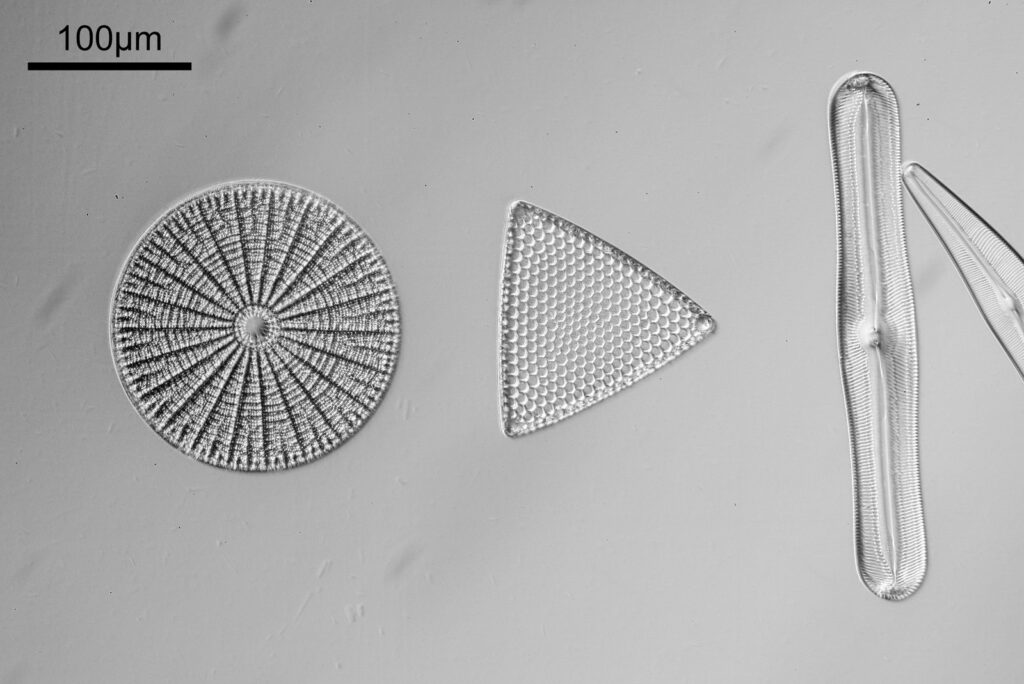
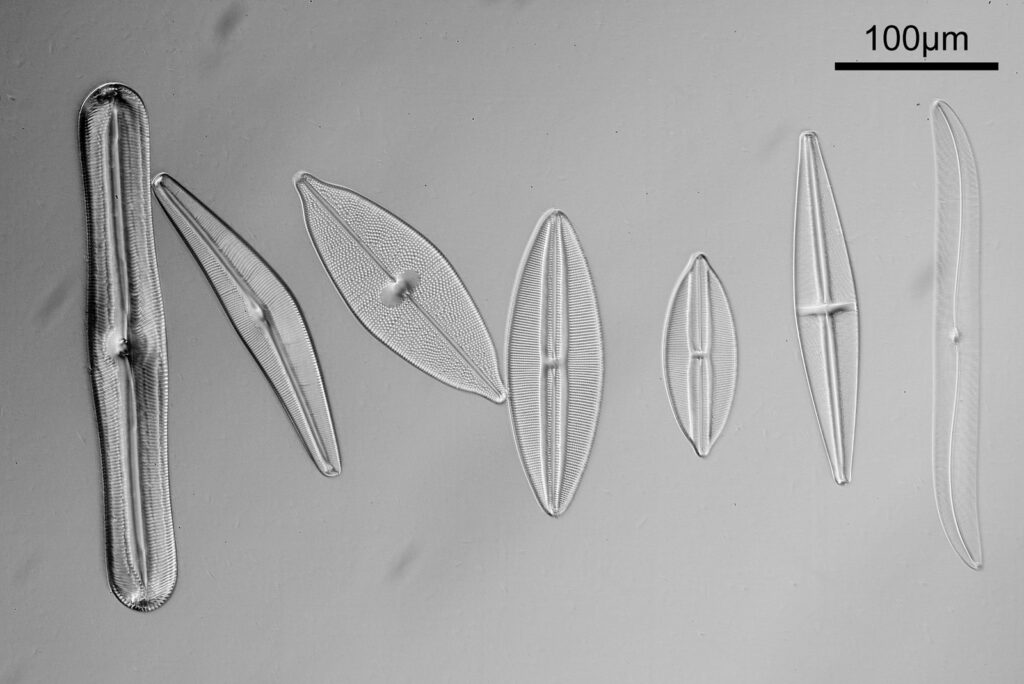
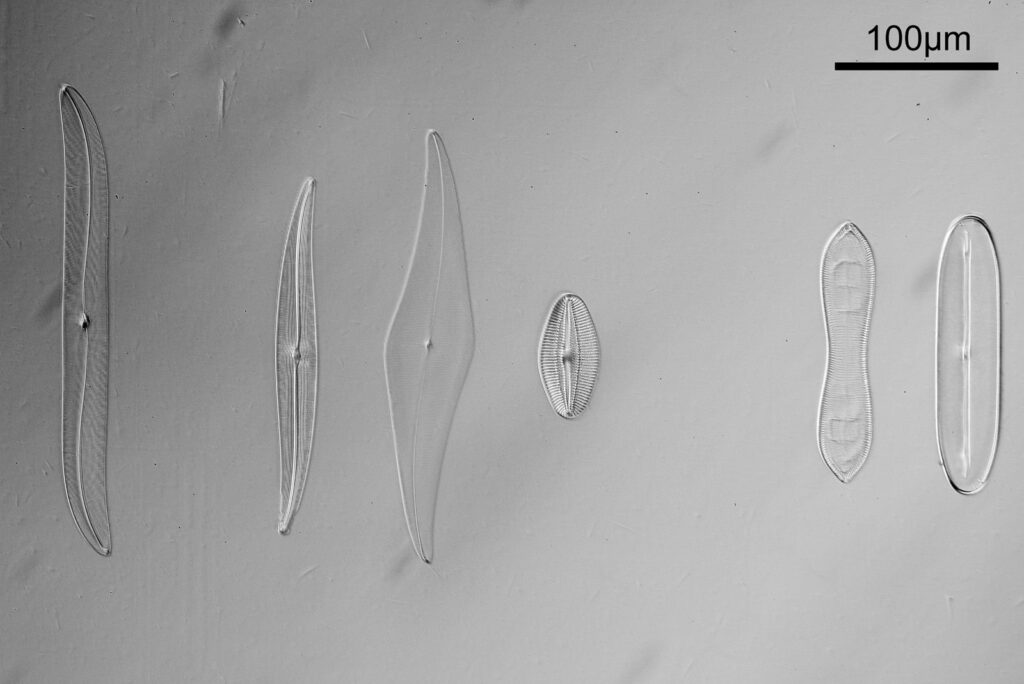
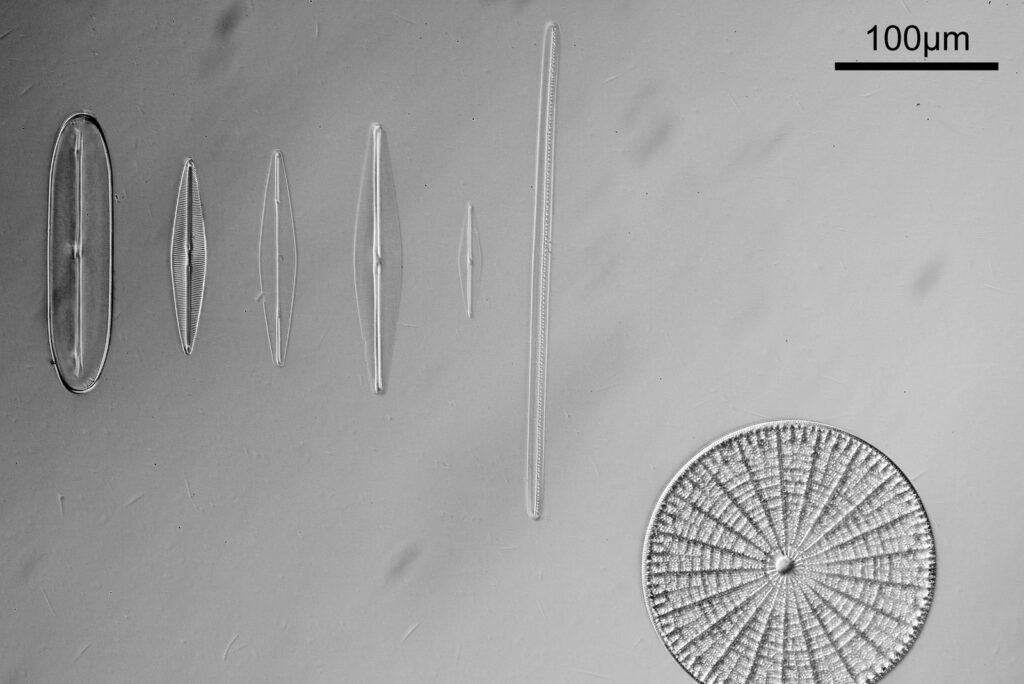
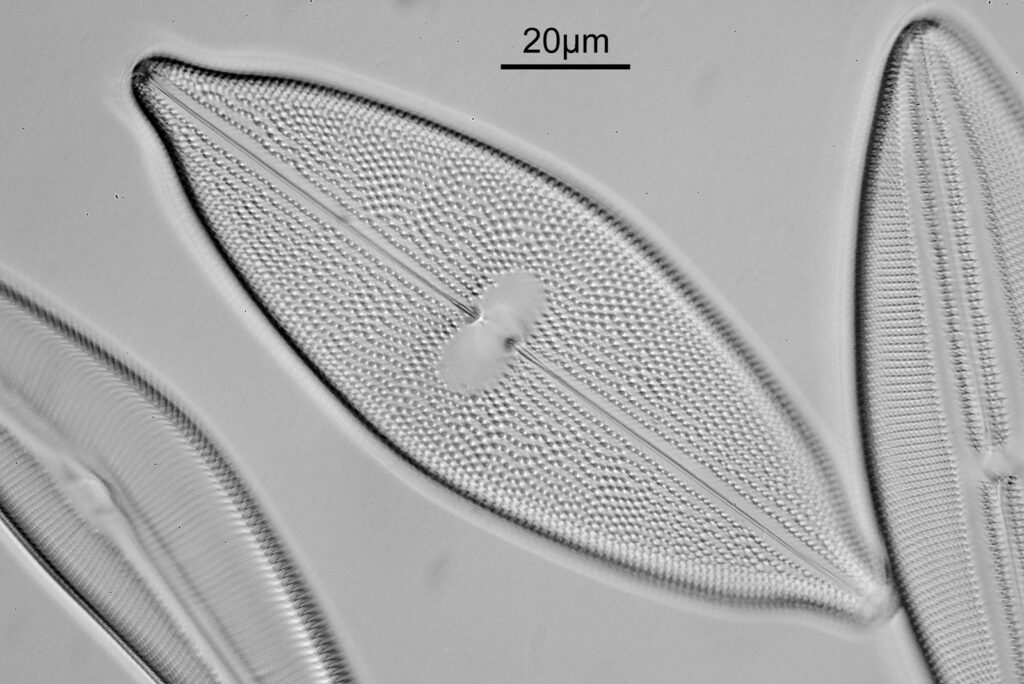
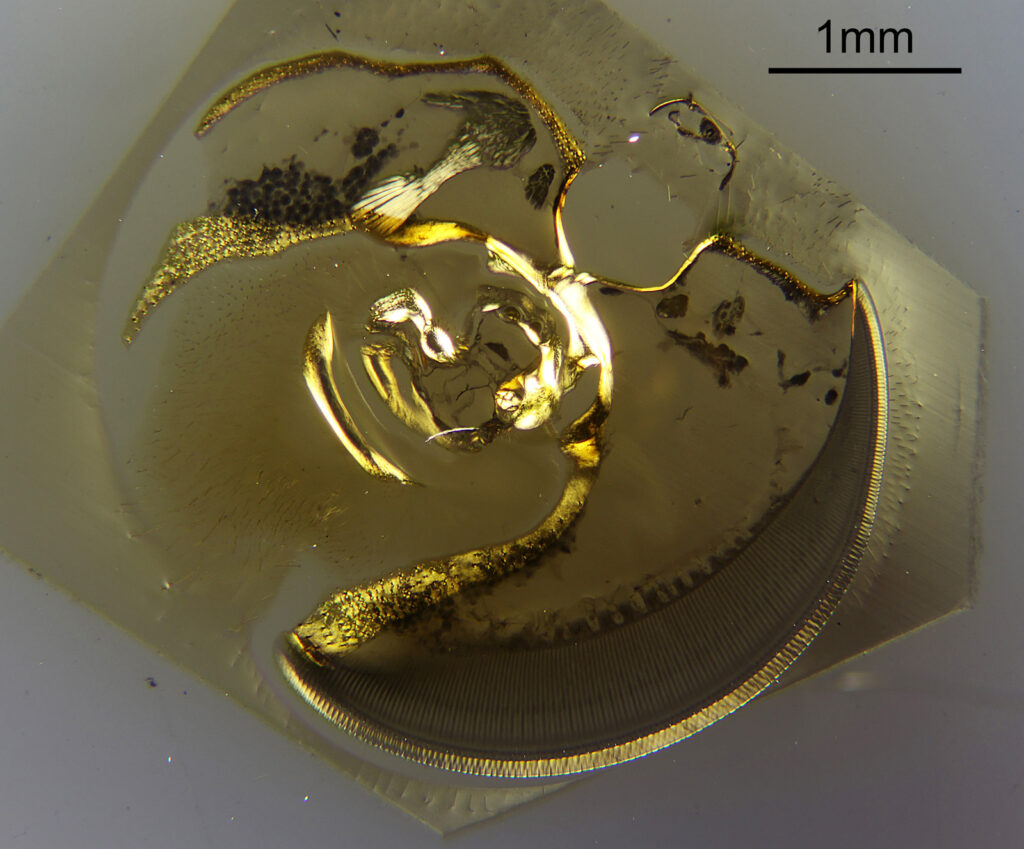
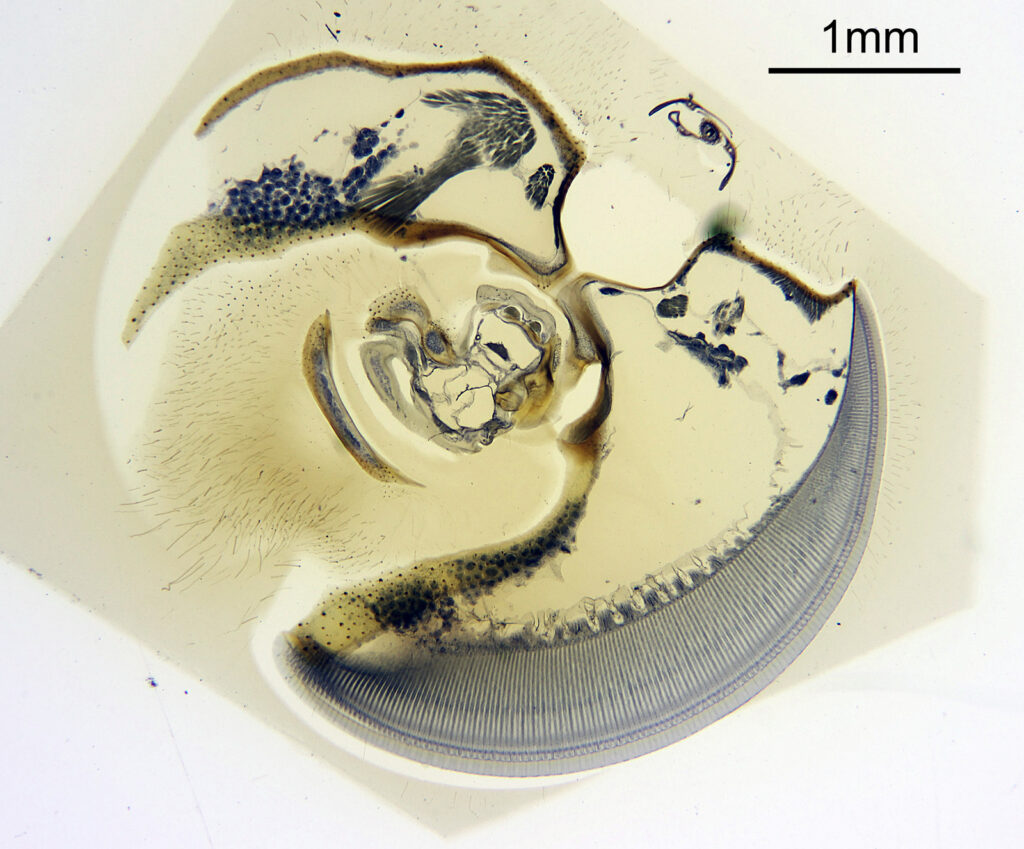
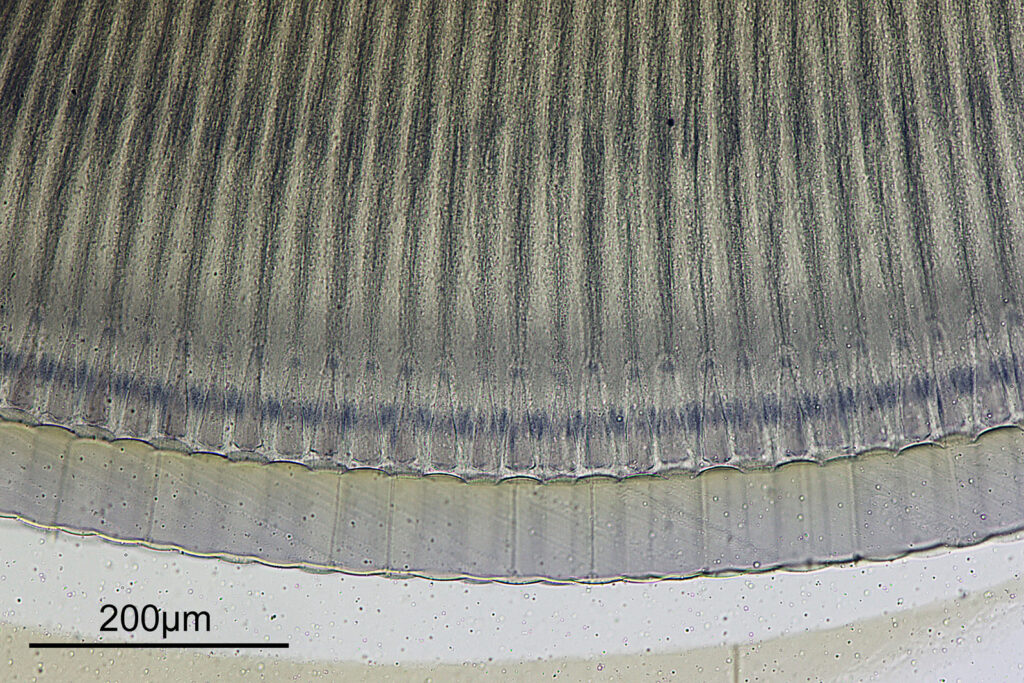
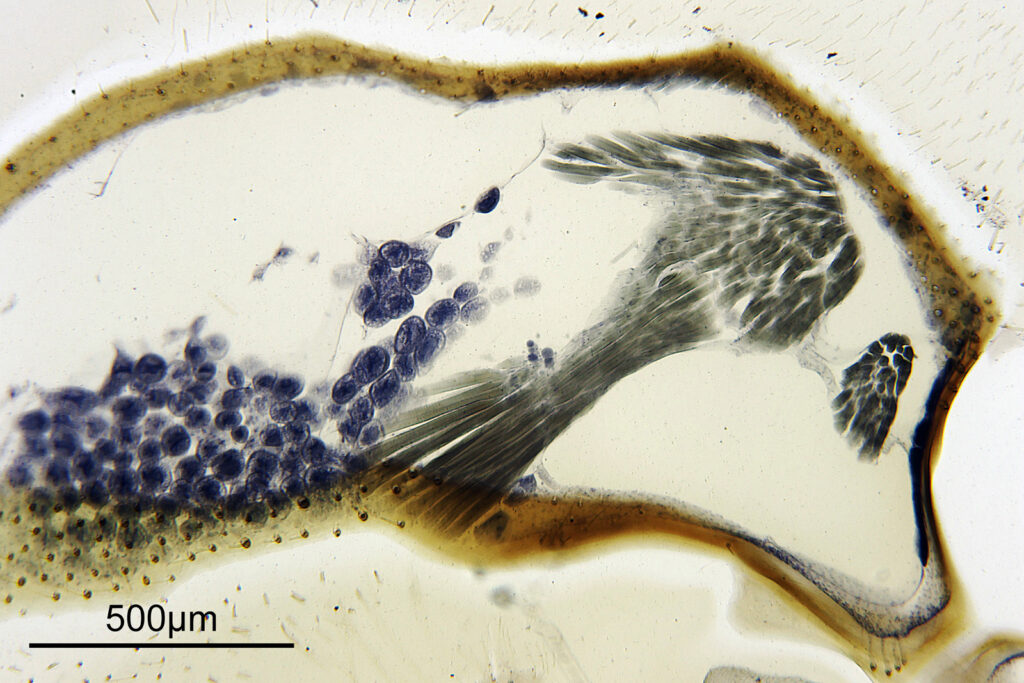
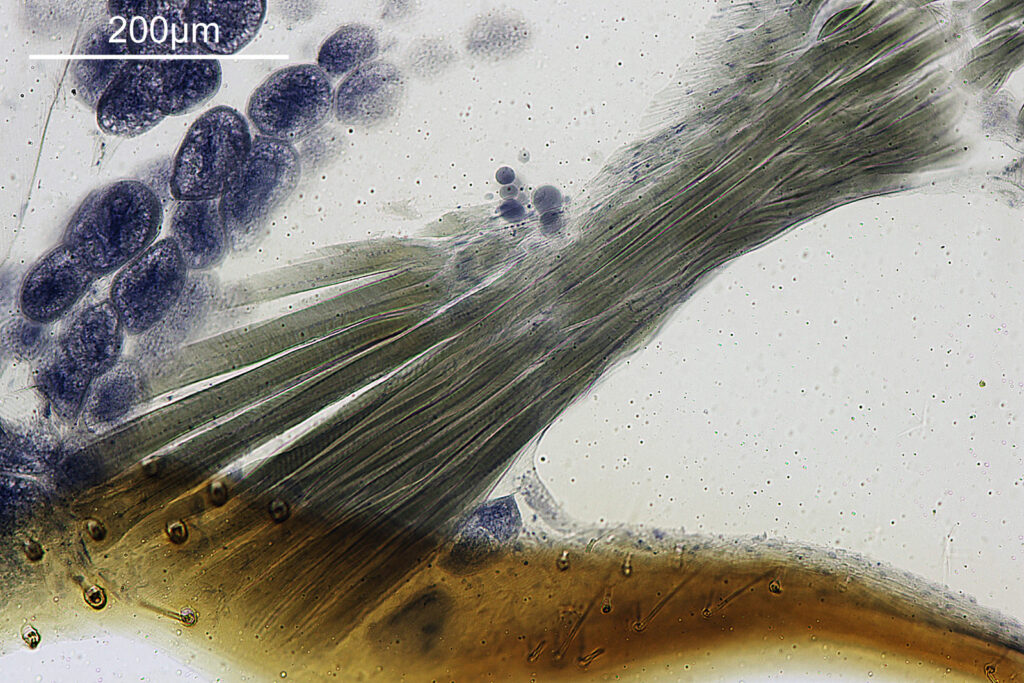
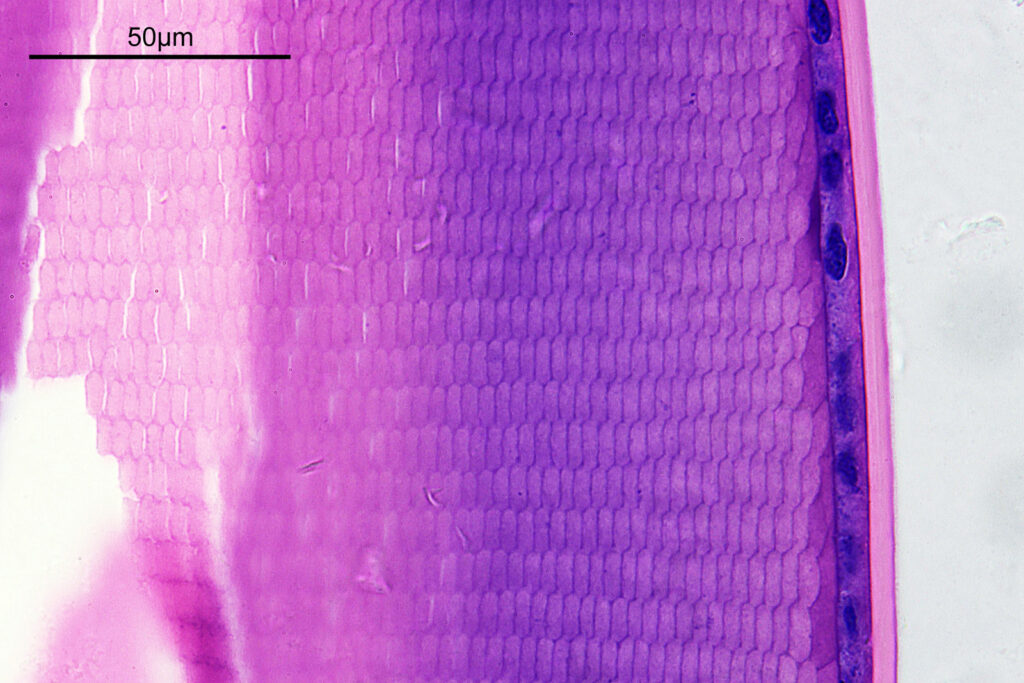
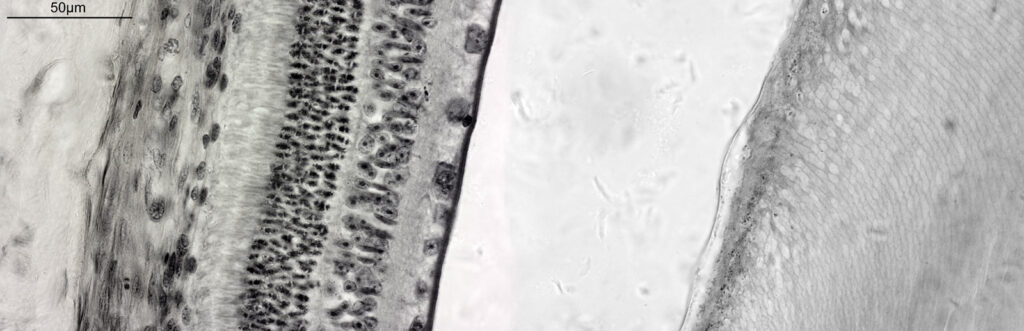
For the image I used an aluminium coated slide by Horace Dall. The slide is marked PA indicating it as Pleurosigma angulatum diatoms. Imaging was done on my modified Olympus BHB using 365nm light from an LED torch, an Olympus Abbe condenser (glycerine immersion), the Nikon 40x UV-F NA 1.30 objective with glycerine immersion, and a Nikon 5x CF photoeyepiece. Light was filtered with an Edmund Optics 365nm, 10nm bandpass filter. Camera was a monochrome converted Nikon d800 by MaxMax. 8 images were stacked in Zerene. The main image has been reduced in resolution for sharing.

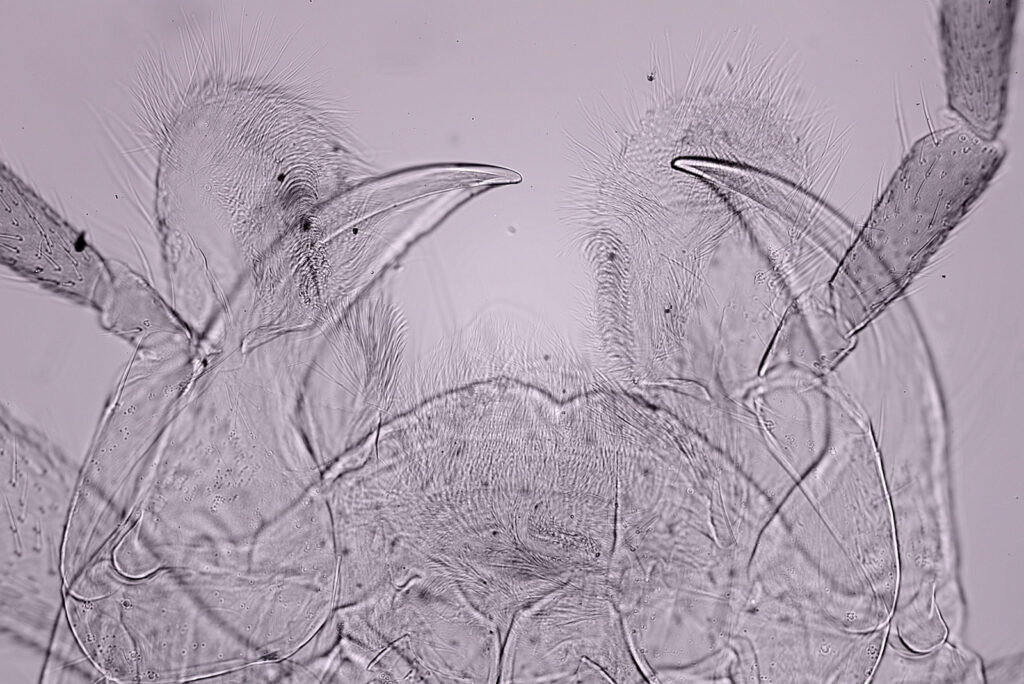
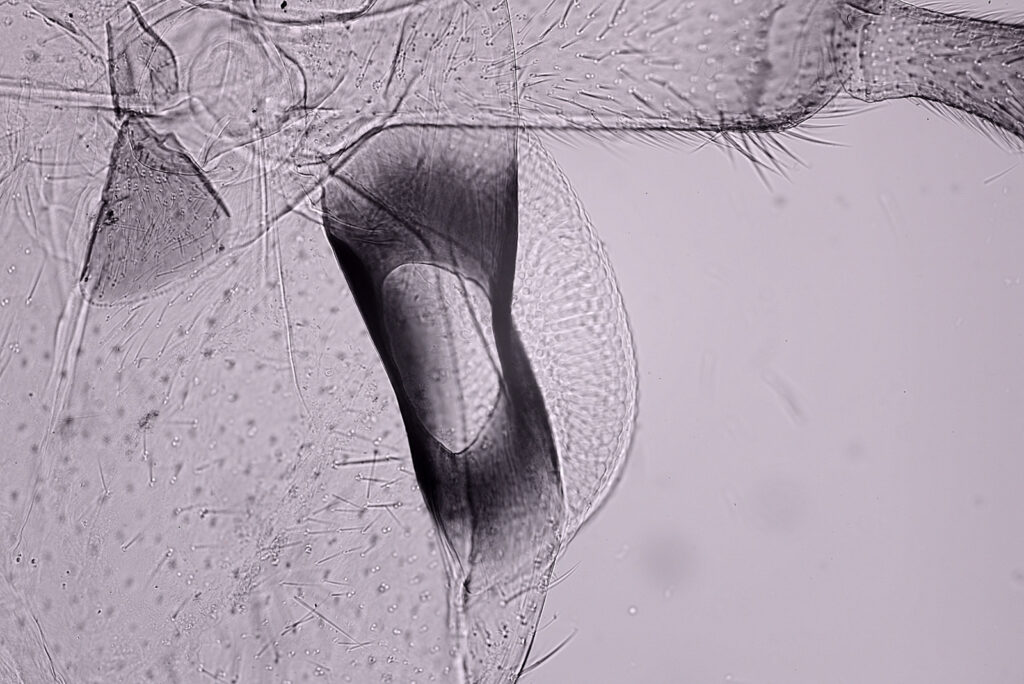
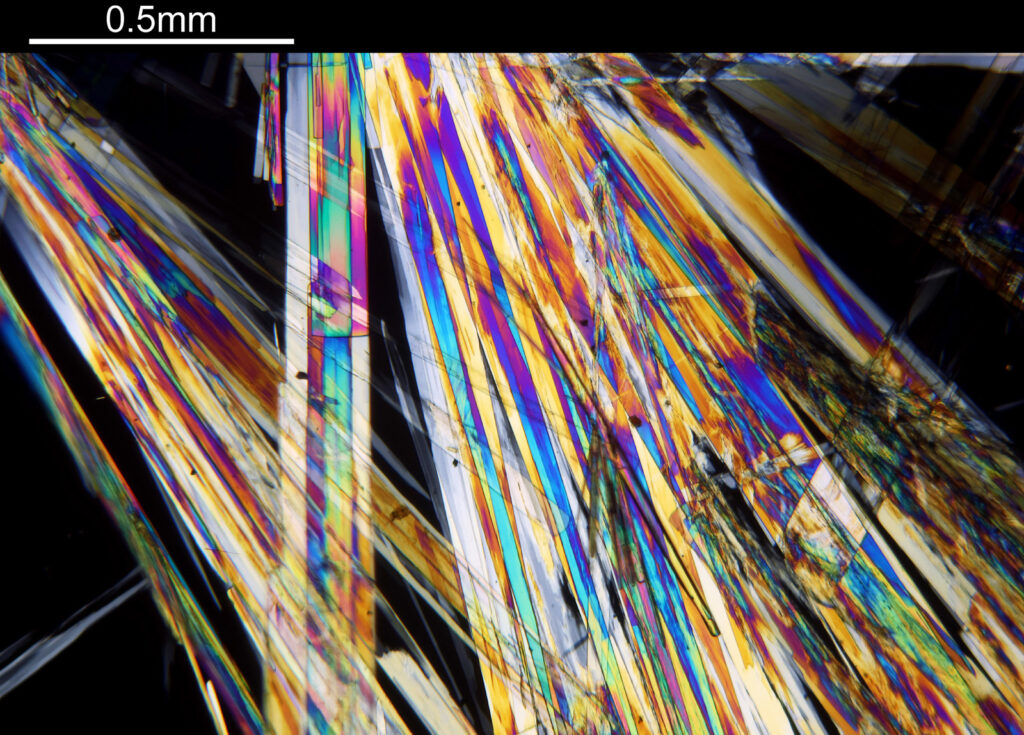
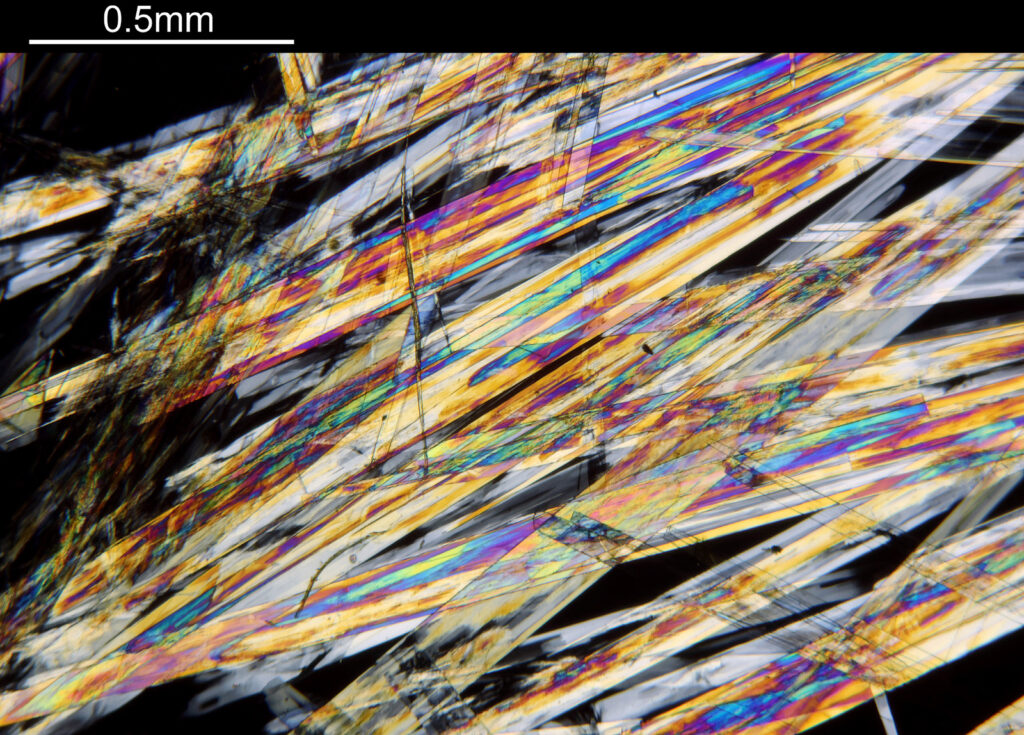
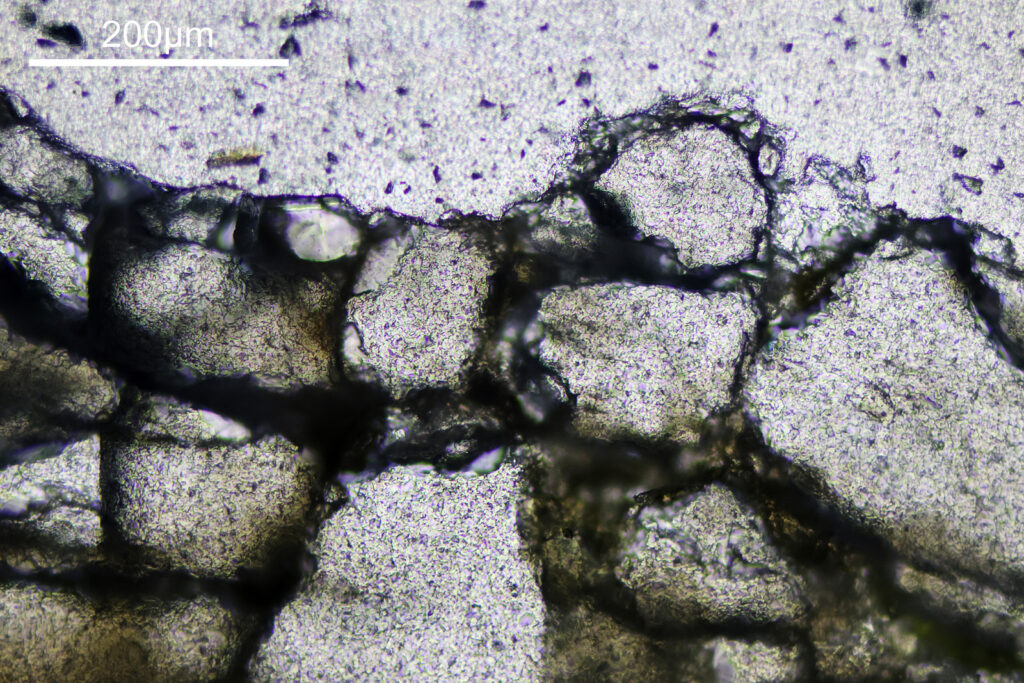
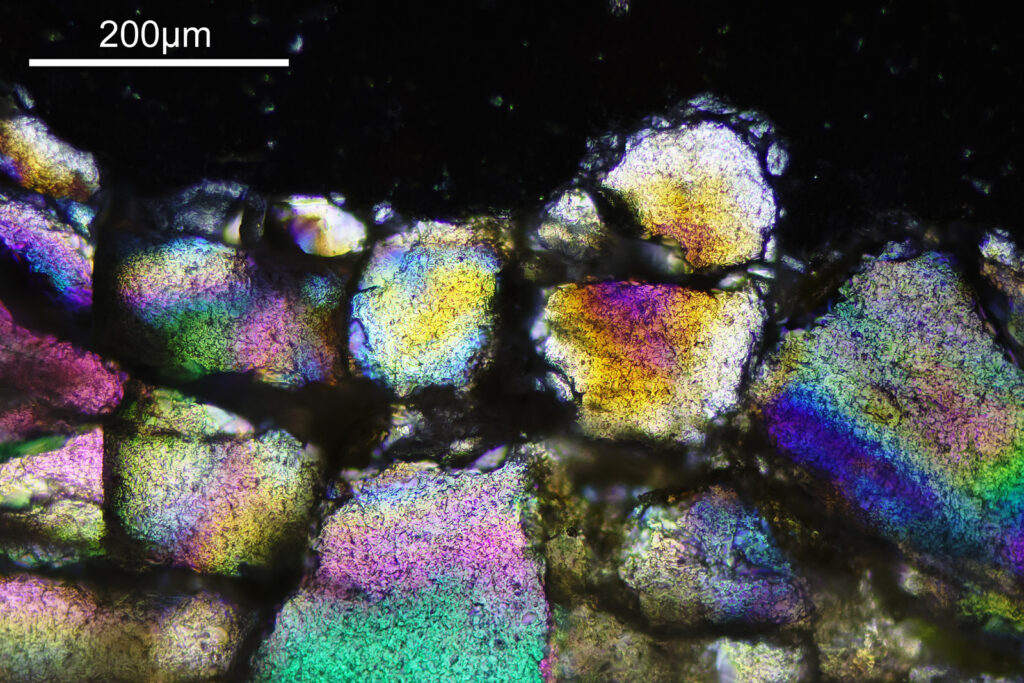
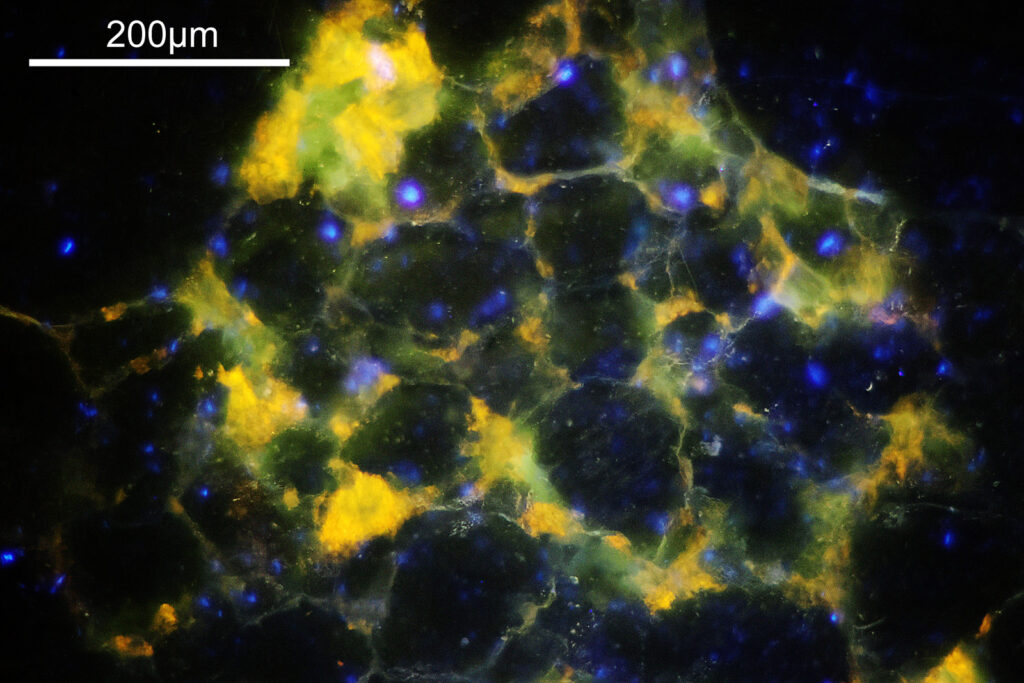
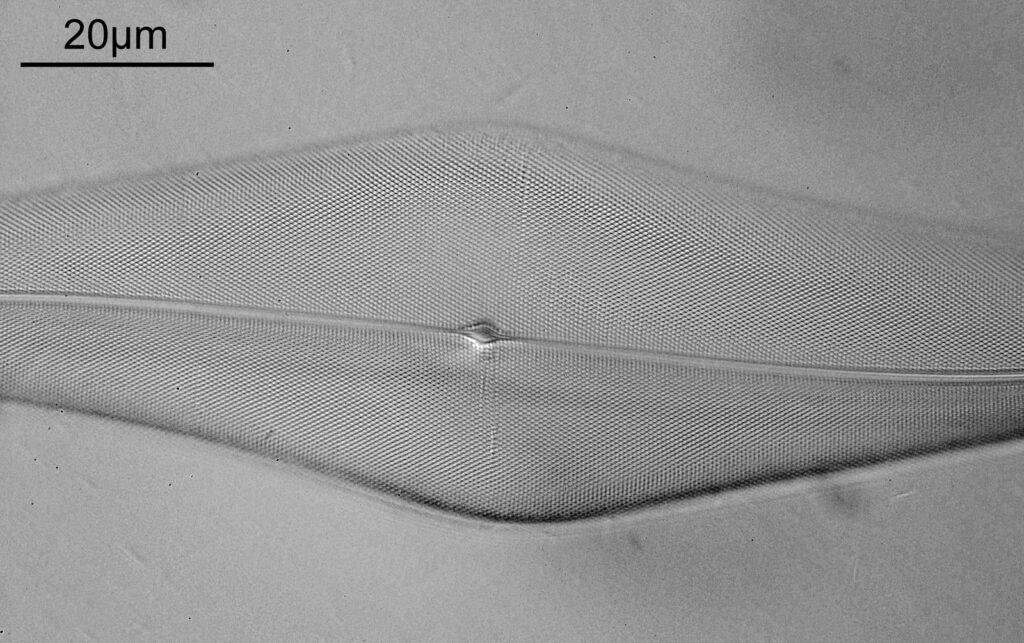
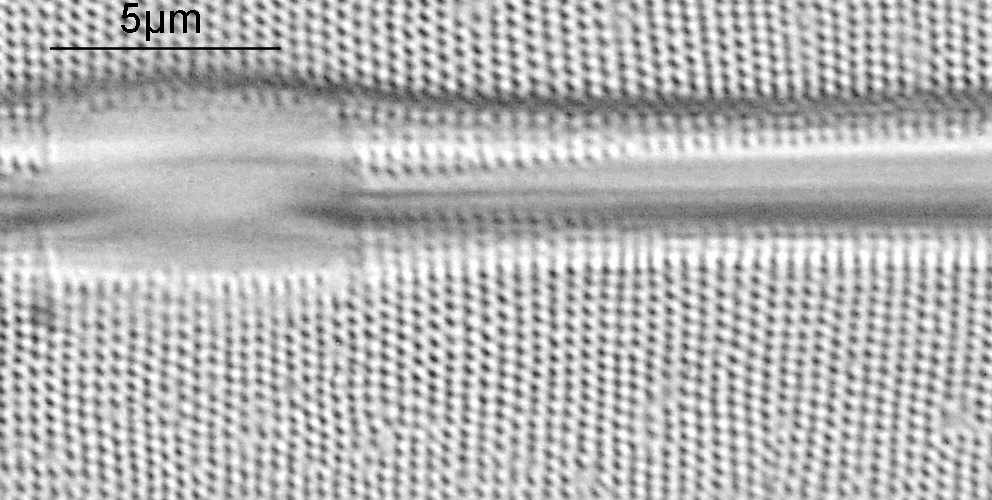
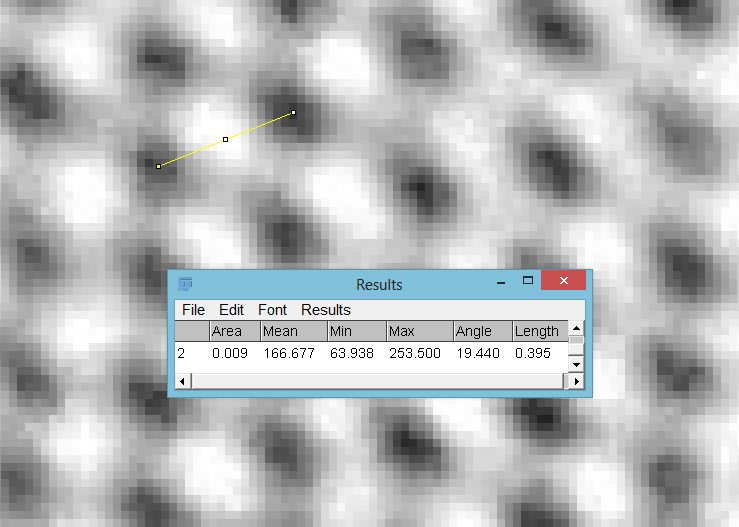
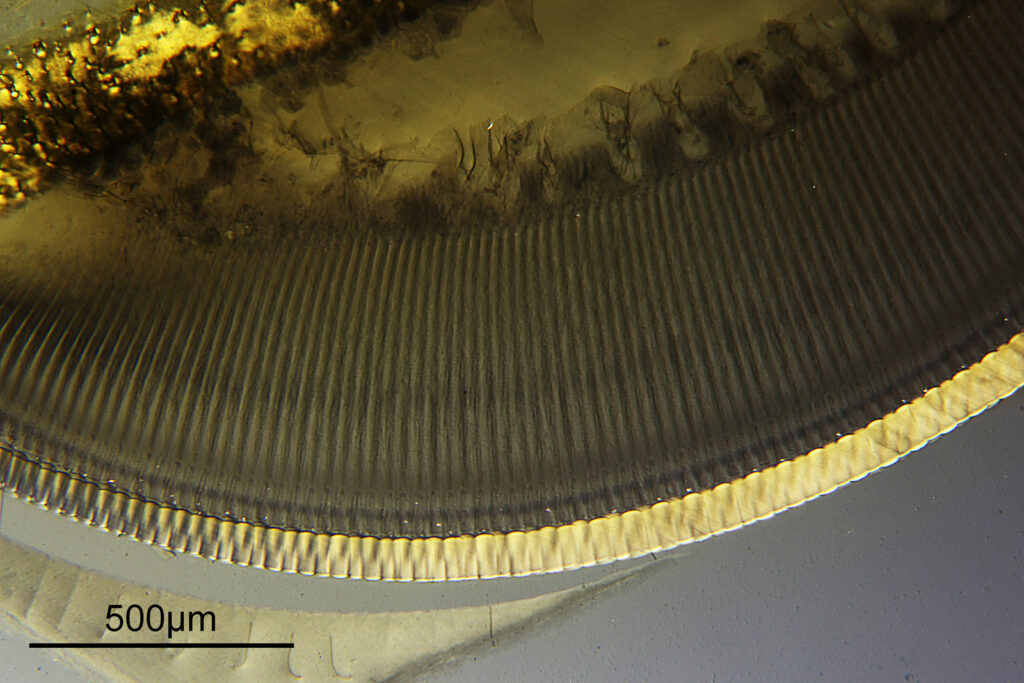
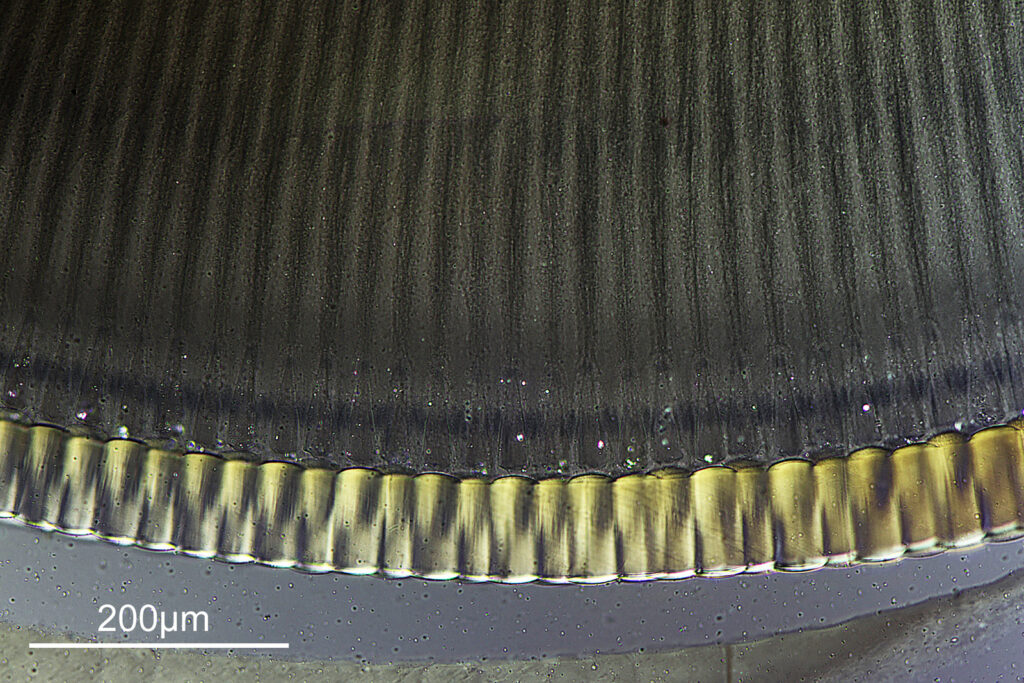
The final image was about 6200 pixels across and has been reduced to 1600 here. The stack produced plenty of detail, and to try and show this better, there are a couple of crops below which are shown at original pixel resolution.
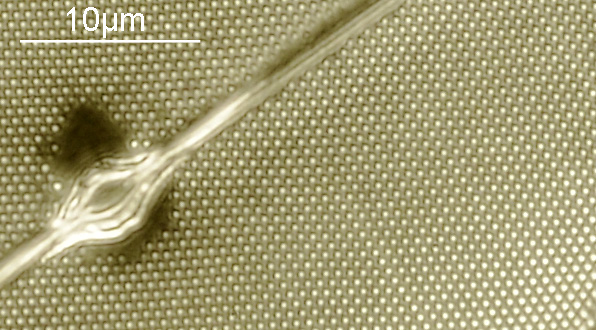
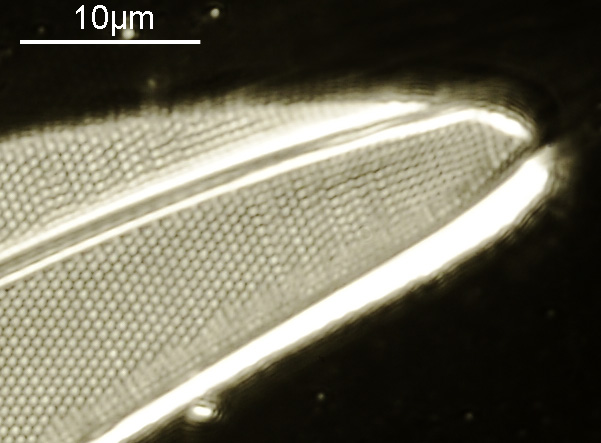
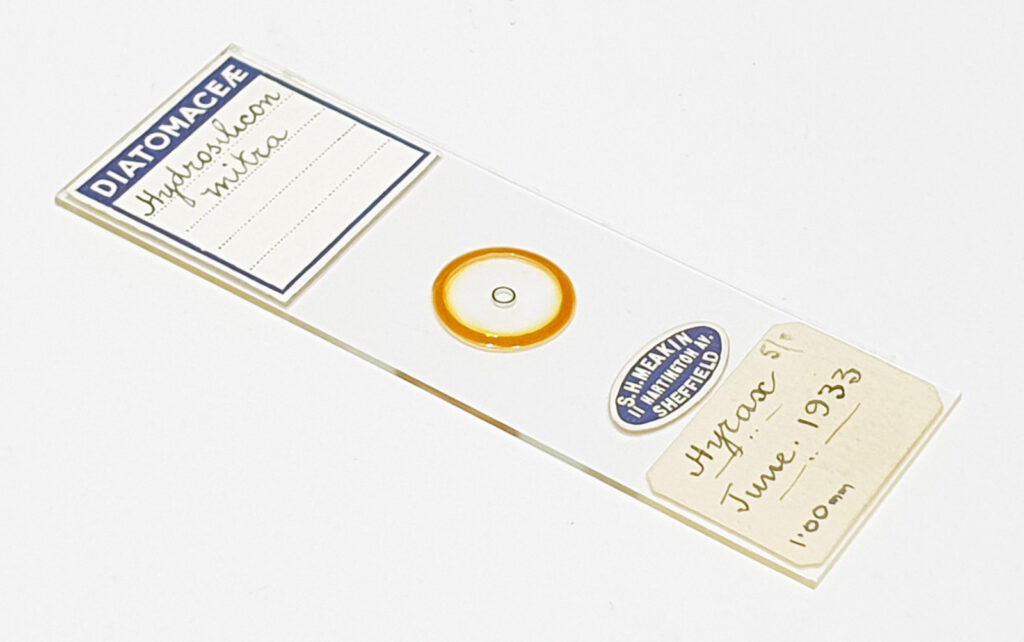
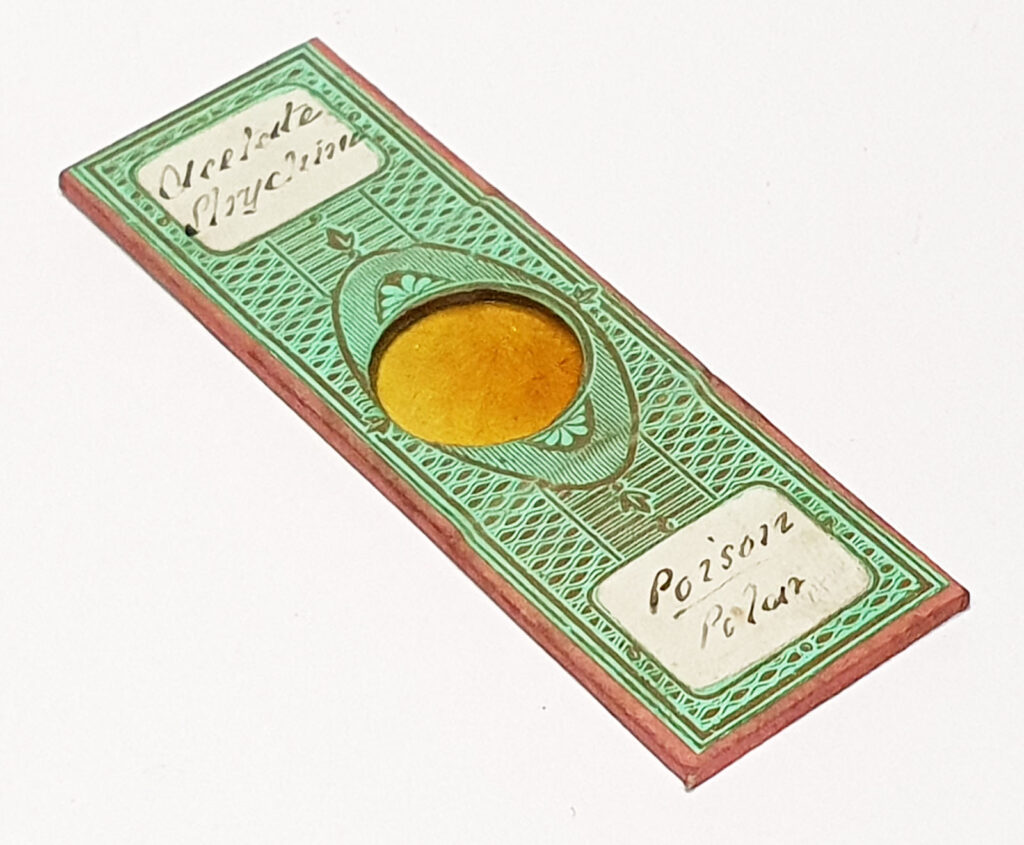
And of course, here’s a photo of the amazing Horace Dall slide.

What about the objective? Here it is.


Why is this a special objective? Firstly, it’s a 40x objective with high NA. It is also quite rare, dating to around the mid to late 1980s. Originally developed for fluorescence imaging work, so it has good UV transmission (for longer wavelength 365nm UV anyway). There’s not much info about these objectives out there, but Charles Kreb’s site shows a Nikon CF brochure which has the 100x version of the objective, here. These aren’t Plan objectives nor are they Apo, so expect some field curvature and chromatic aberration if not using monochromatic light. What they are designed for is the let a lot of light through, and they certainly do that.
I have a 100x version of the objective, which has also been used for imaging at 365nm. The 100x is nice as it has an adjustable iris making it great for darkfield. Here are the 40x and 100x together.

If anyone has the 10x and 20x from this series that you are looking to sell, please let me know as I am on the lookout for those two for my UV work.
We often do not thank our suppliers, but they make the work we do possible. As such, thanks again to JB Microscopes – great customer service and worth contacting if you are in need of something specific (they do not list their stock on their site). As always, thanks for reading, and if you’d like to know more about my work, I can be reached here.