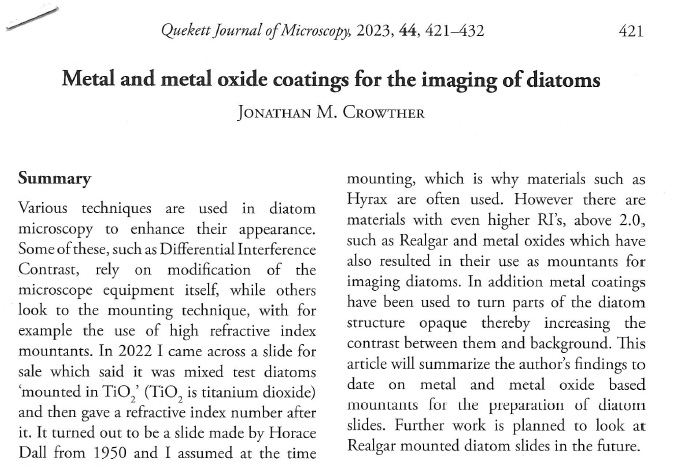
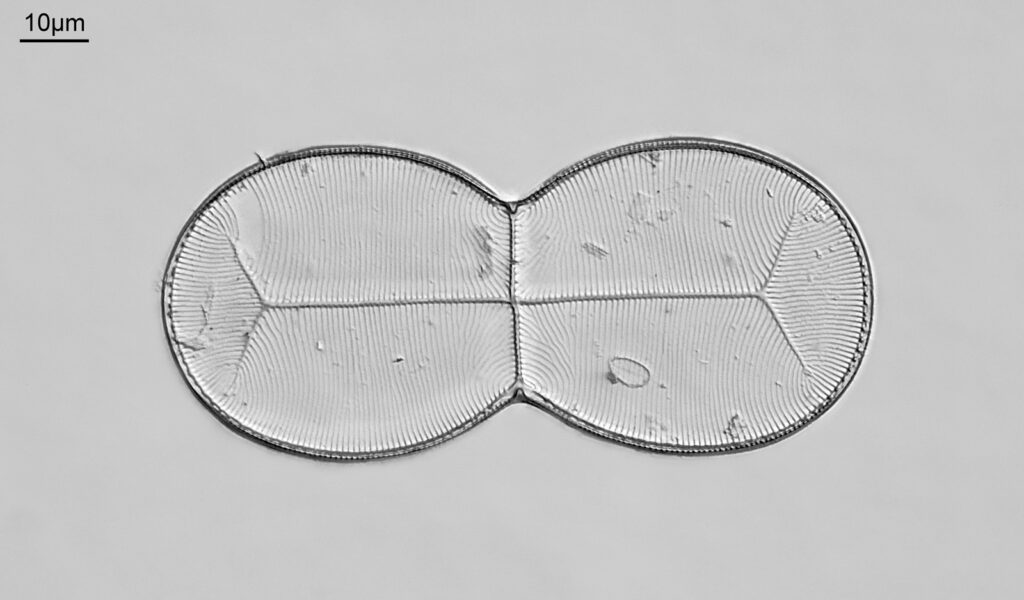
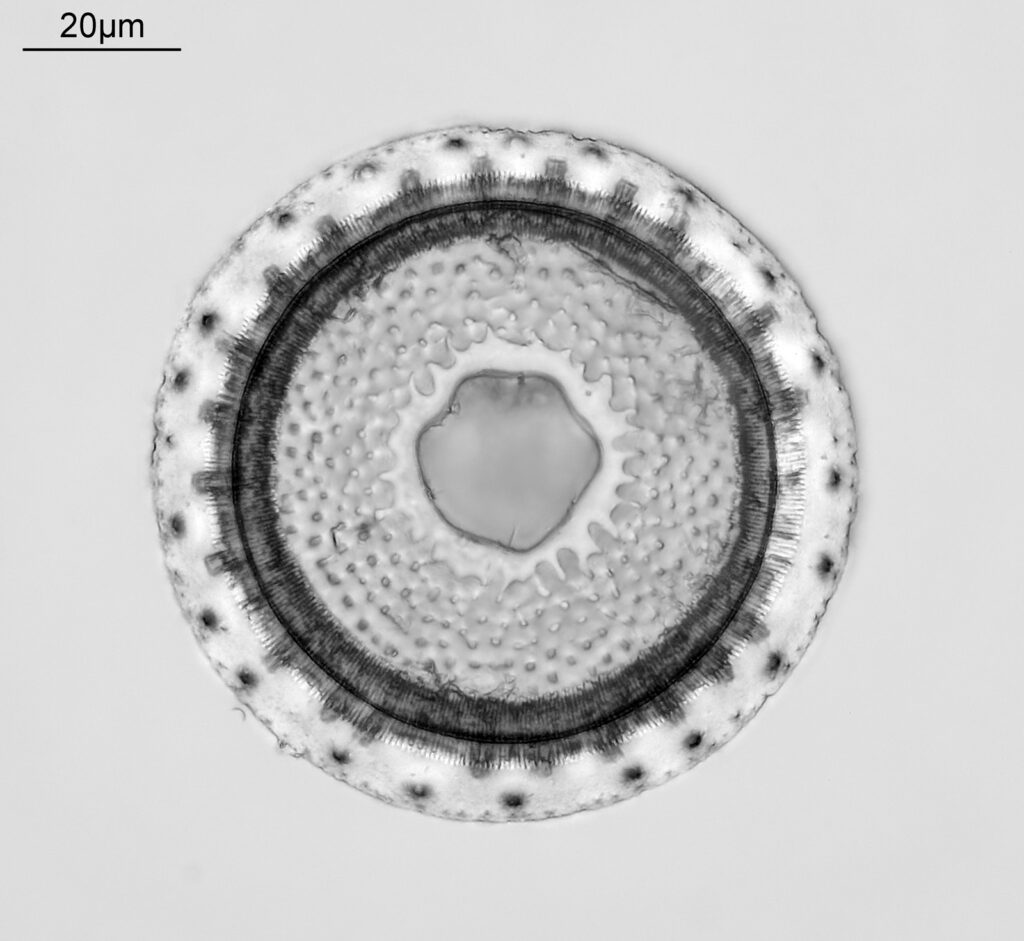
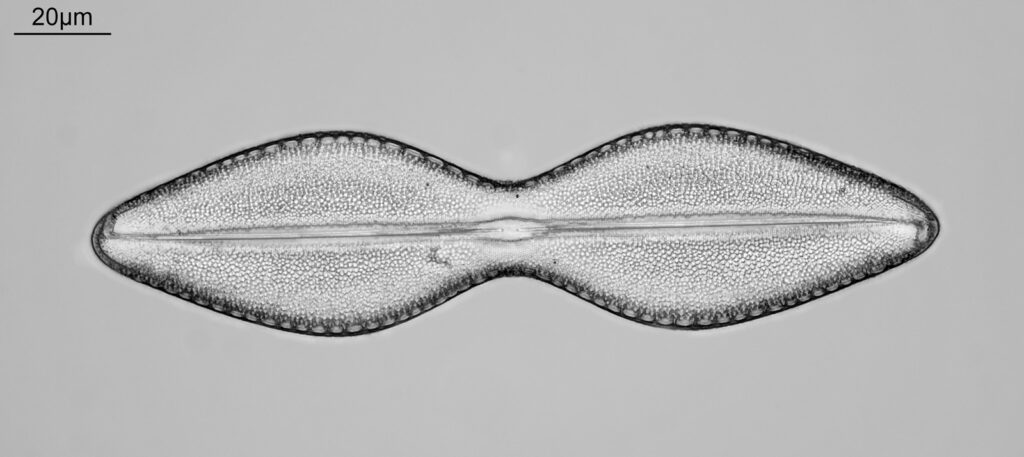
Firstly, a belated Happy New Year to everyone. I wish you all the best for 2024. I start the year with some microscopy work, but something a little different to the diatoms I regularly share. My day job is in the field of dermatology, and the initial reason I built my UV microscope was to be able to image sunscreen (SPF) products. Specifically to be able to image UVA and UVB sun filters separately and see where they are in the cream. While most of my imaging is done using photographs I do occasionally do video work. This post shares some really funky videos looking at titanium dioxide (TiO2) particles in a partially dried sunscreen film, with the videos being recorded both using UVA – 365nm light – and UVB – 313nm light.
The product contained titanium dioxide (TiO2) particles as the UV blocking ingredient. The slide was prepared and the product allowed to dry slightly before imaging. Imaging was done using my modified Olympus BHB microscope with a 100x Zeiss Ultrafluar NA 0.85 objective lens, and using two different UV wavelengths – 365nm (UVA) and 313nm (UVB). This is not fluorescence imaging, this is direct imaging of the UV at the different wavelengths.
Two videos to share. First, captured using UVA light.
What are we looking at here? The video shows a partially dried down film of a sunscreen cream. There are regions where there are still droplets of oil from the topical emulsion before the droplets have chance to coalesce. Inside the oil droplets there are particles which are moving – these are particles of TiO2. Some of these particles look white and some look black in UVA, i.e. some are absorbing the UVA and go black, and some are reflecting or scattering the UVA, and look white. Whether they absorb or scatter a given wavelength depends on their particle size. However the really cool thing is that they are moving and moving quite a lot. What’s causing this? I’m shining UV on the slide with the product film and some of that is being absorbed by the TiO2. As a result it will warm up and start to move – Brownian motion – bouncing into other particles and transferring energy causing them to bounce around. As a result UV energy is absorbed resulting in particle motion.
With my microscope I can image other UV wavelengths. By swapping filters and imaging at 313nm I got the following video.
Same region of the product film, but now we have a video of how the sample looks using UVB light of 313nm. The video is much grainier as the camera is less sensitive at that wavelength, so the ISO needed increasing to be able to capture video footage. The particles look different now – most look black showing that most are now absorbing the 313nm wavelength light rather than scattering it. This is to be expected for TiO2 particles as the absorption of light is wavelength dependent. The motion is still present. The particles are typically sub-micron in size, but are probably aggregations on individual TiO2 particles as the smallest ones would be too small for me to resolve on my microscope.
Before I wrap up, I want to revisit the second video. Excuse me while I geek out for a moment. This was imaged using 313nm light and through a high magnification microscope objective. The camera was a monochrome converted Nikon d800 prepared by MaxMax in the US. Even though monochrome conversion improves sensitivity at 313nm, I was amazed to be able to capture live video of the behavior of this sample and how it looked in the UVB region.
It’s funny, when I first looked at these samples the movement I observed reminded me of cells moving around. However as I mentioned above, this is about energy transfer and heating of inorganic particles. The motion, while cool to see, is actually a problem for capturing images. Often my exposure times for capturing UVB microscope photos are of the order of half a second or more. Movement of the particles causes blur and reduces the resolution achievable. As always it is important to understand the techniques you are using when trying to understand what it is they are telling you.
I’d like to thank DSM-Firmenich AG for supplying the sunscreen sample to image, for funding some of my UV microscopy research and for giving me permission to share these videos. As always, thanks for reading, and if you’d like to know more about my work I can be reached here.