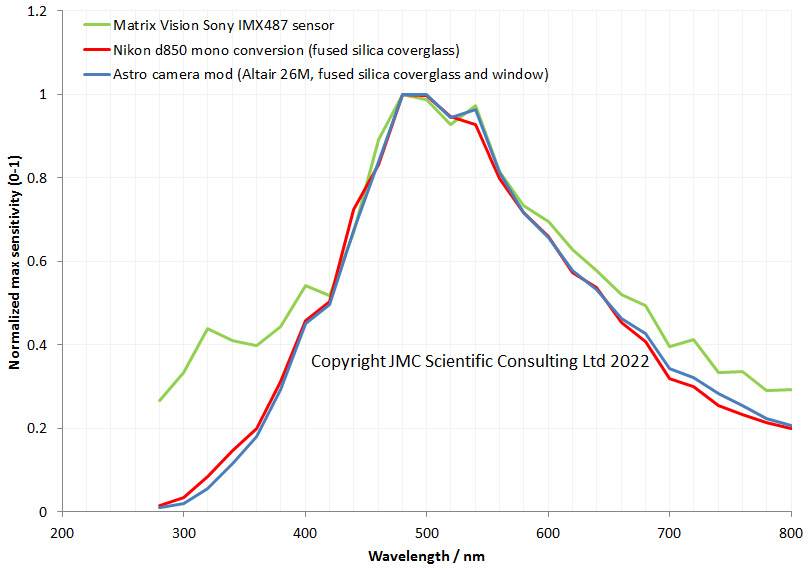
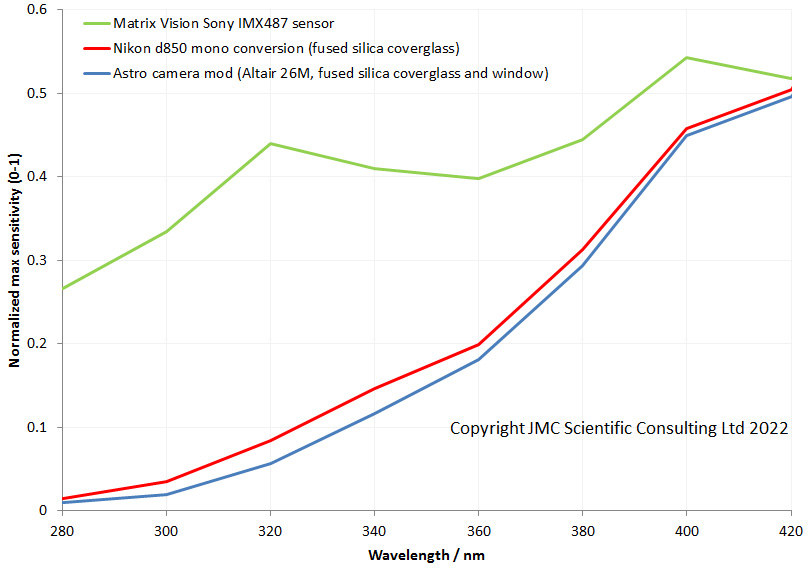
My new UV camera uses a Sony IMX487 sensor, and as I showed recently certainly offers improved UV sensitivity, and its behaviour at 280nm is very impressive (as I have shown here). With my spectral sensitivity testing though, I can only get down to 280nm. With the UV microscope I built, one of the goals was to use it for imaging at 254nm (see here for some early work on that), however at the time, my cameras had very little sensitivity that far into the UV and I was dealing with very long exposure times, often 10 minutes or longer. Another issue was that live view didn’t work so focusing was a nightmare. Today I will share some initial images taken with the new Matrix Vision camera with the Sony IMX487 UV sensor.
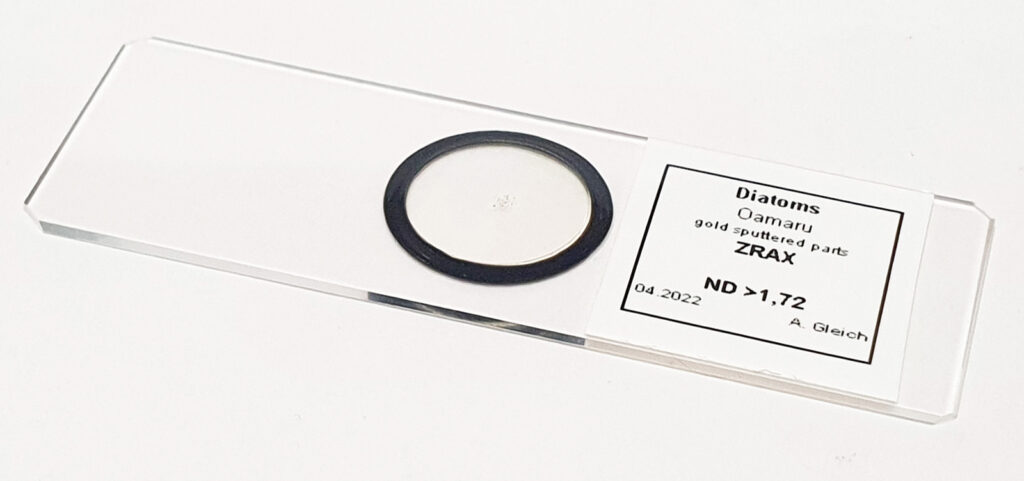
The slide imaged was my custom made diatom slide (made with quartz and fused silica instead of glass for the slide and coverslip) on my modified Olympus BHB microscope, and using the 254nm lamp I built. The objective was a 40x Leitz NA 0.65 UV objective with glycerine immersion and the condenser an antique Zeiss quartz one. The optical filter was a 254nm one from a forensics camera. The images are single shots (not stacks) and show parts of two diatoms on the slide. They are shown as full frame images and are not cropped, although I dropped the resolution of the images from 2848×2848 to 1600×1600 for sharing here. They have been sharpened slightly, and I played with the curves a bit as well.
First one, part of a Pleurosigma angulatum diatom.
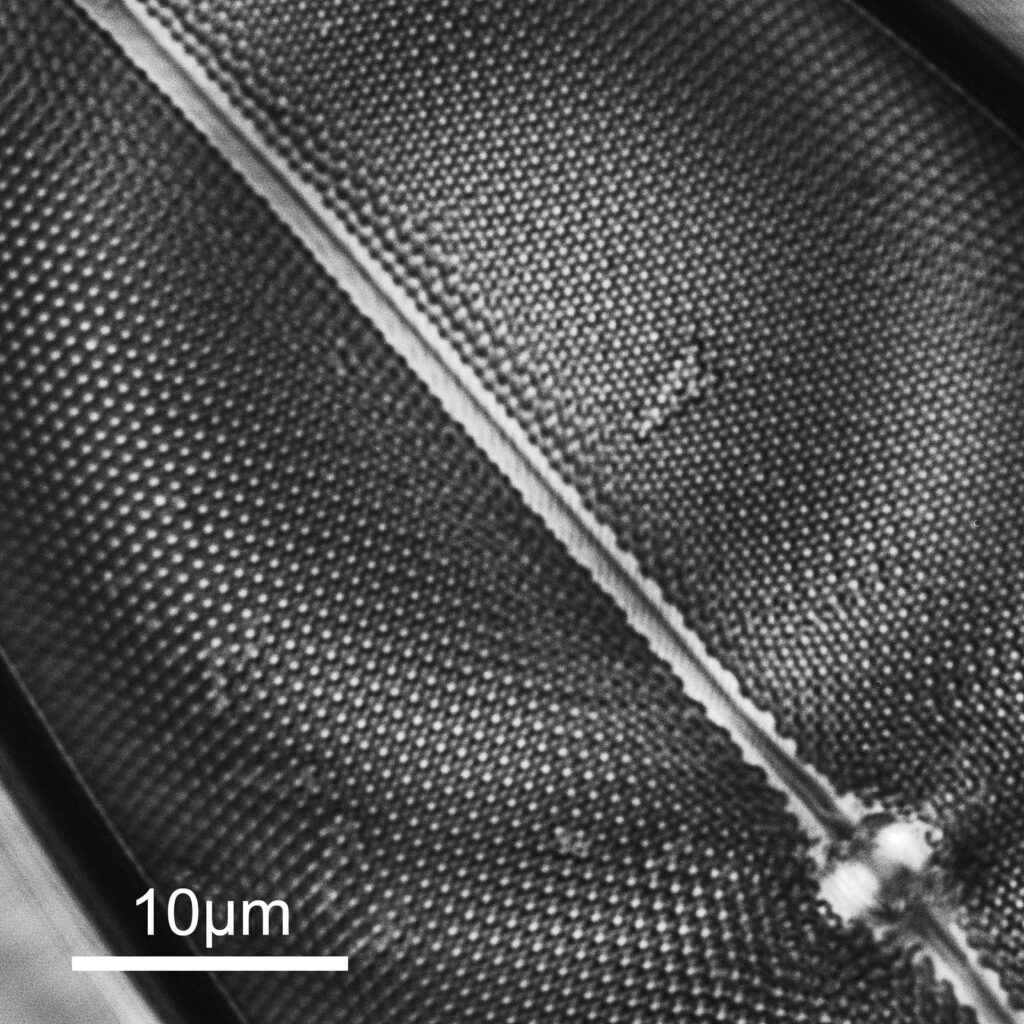
And second one is part of another diatom (I am not sure what this one is).
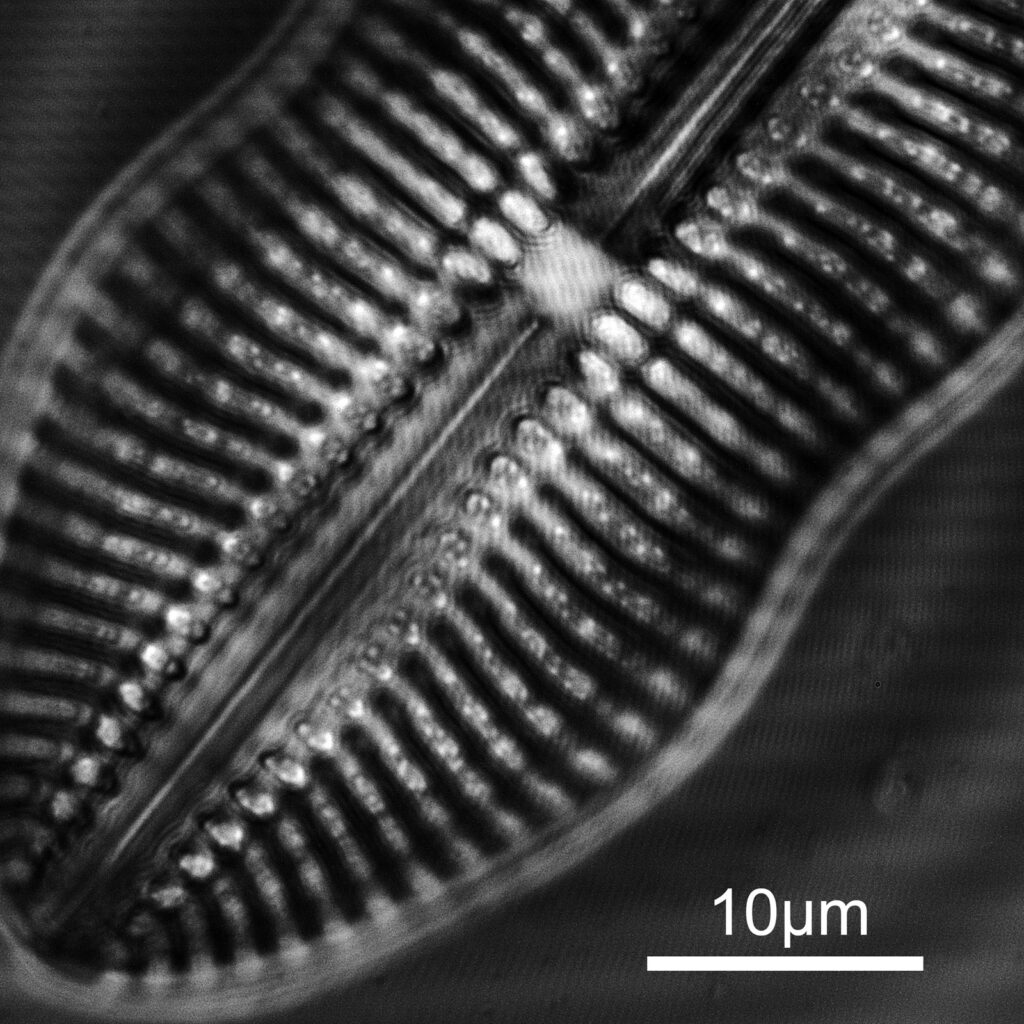
The images are showing very high resolution – which is what would be expected given the short wavelength – especially given that the NA of the objective is only 0.65. There are some artifacts in the images (banding for instance) and I am not sure how much this is due to the camera settings, the light source itself or other optical components in the setup as I have seen similar effects before with another camera at this wavelength. I shall have to dig more into that. On the second image there are some very bright white points of light which are appearing in the structure of the diatom. I’ve not seen these before, but they seem to be ‘real’ features and not artifacts. This is what happens when you look at things with different wavelengths…..
With this new camera I was able to capture images in seconds rather than minutes (14s exposure and a gain of 20 were used for these images as opposed to 10-30mins and ISOs of 4000 and above) and I was able to focus using live view on the computer so it is definitely an improvement. Id like to be able to use lower gain for the images, but that means longer acquisition times than the software currently allows for, so is not a simple ask. The sensor is much smaller than I am used to with my SLR cameras (11mm x 11mm vs 36mm x 24mm) and finding the diatoms was a challenge even with this 40x objective. I dread to think how difficult it would be with a 100x one.
As far as I am aware these must be some of first images with this sensor (which aren’t from Sony) which are being shared. I hope to do more with this camera for imaging diatoms and also for my sunscreen work, and it continues to impress me with its capabilities. Before I go, a photo to compare the Matrix Vision camera with one of my SLRs (both with UV lenses on), just to show how small it really is.

As always, thanks for reading and if you’d like to know more about this or my other work, I can be reached here.