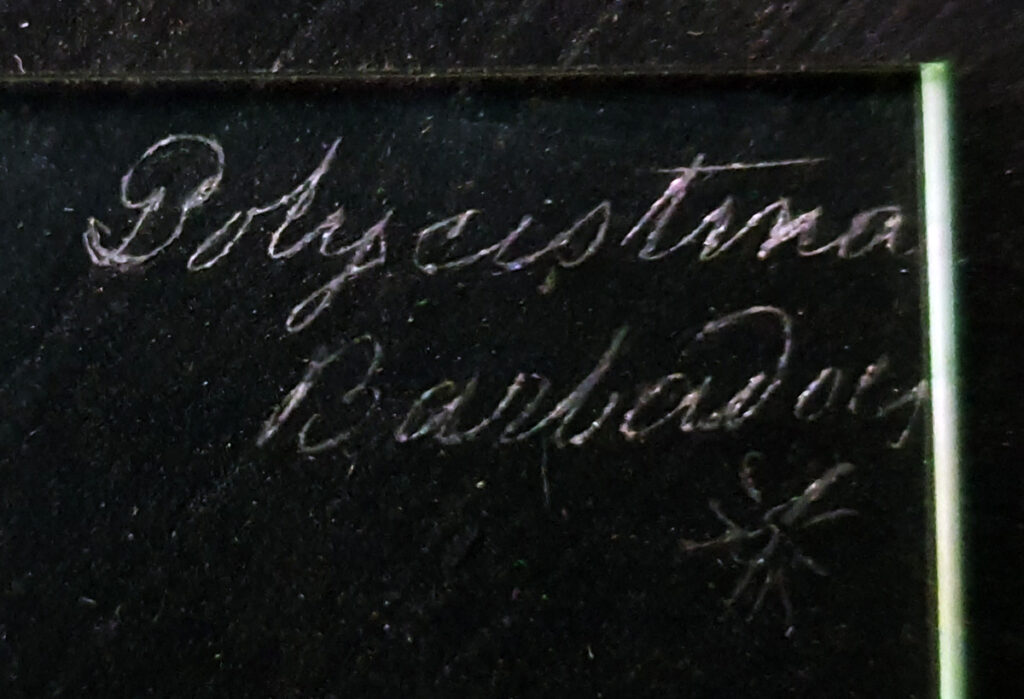It’s no secret that I enjoy imaging diatom microscope slides. They provide delicate and detailed structures and push me to get better at the imaging process. I tend to buy second hand older ones to image. While there are vendors still making slides, I think the older ones tend to have more character, and many are still surprisingly clear and usable for a fraction of the price of newer ones. Today’s post shows some of the slides to arrive recently and discusses how the images were captured (note that images have been reduced in resolution for sharing here, and all have been tidied up a bit to get rid of dust/dirt from the camera sensor).
The first is a slide with a Triceratum kinkerianum diatom mounted in Styrax.
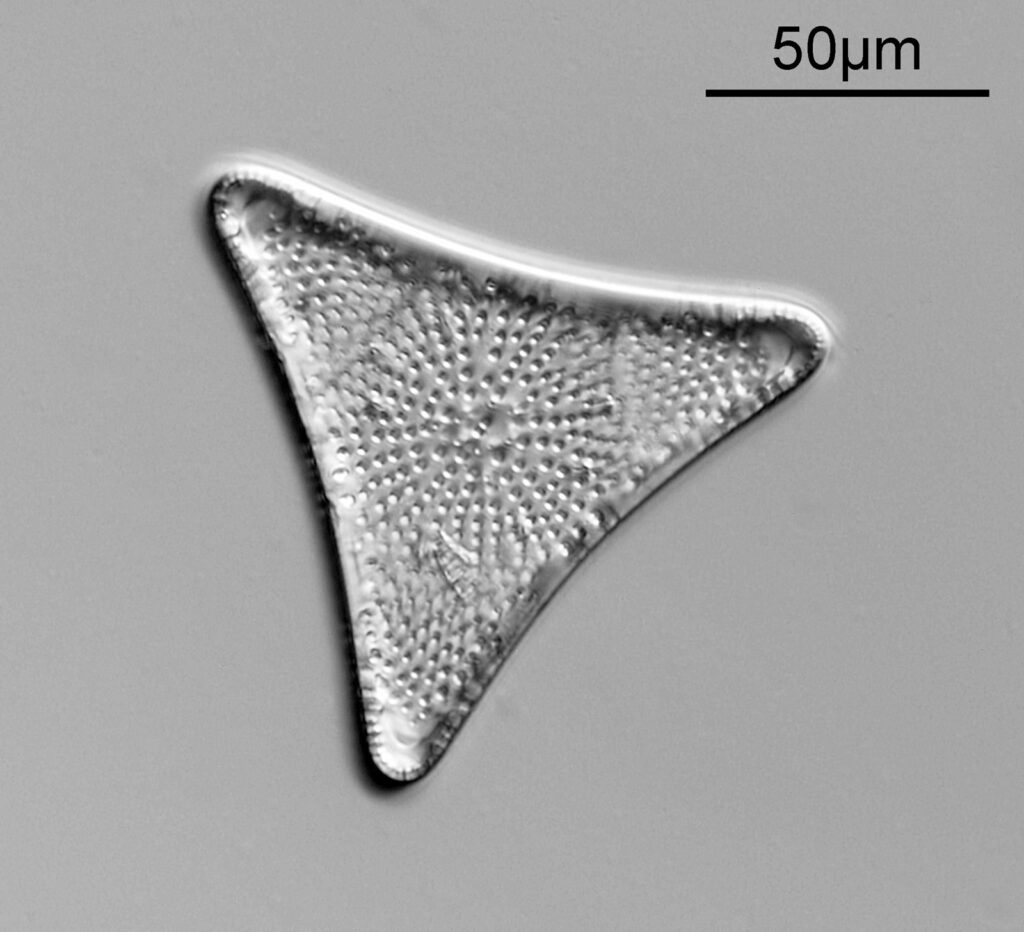
There is only a single example on the slide, so it took some finding as it is very small. This was lit using oblique lighting from below (Olympus Aplanat Achromat condenser) with the condenser set to still produce a bright field image, but one which has obvious side lighting, as can be seen by the shadows in the image. The objective used here was a 20x Olympus Splan NA 0.46, and the camera my Nikon d800 monochrome conversion. White LED light was used. Here’s the slide itself.

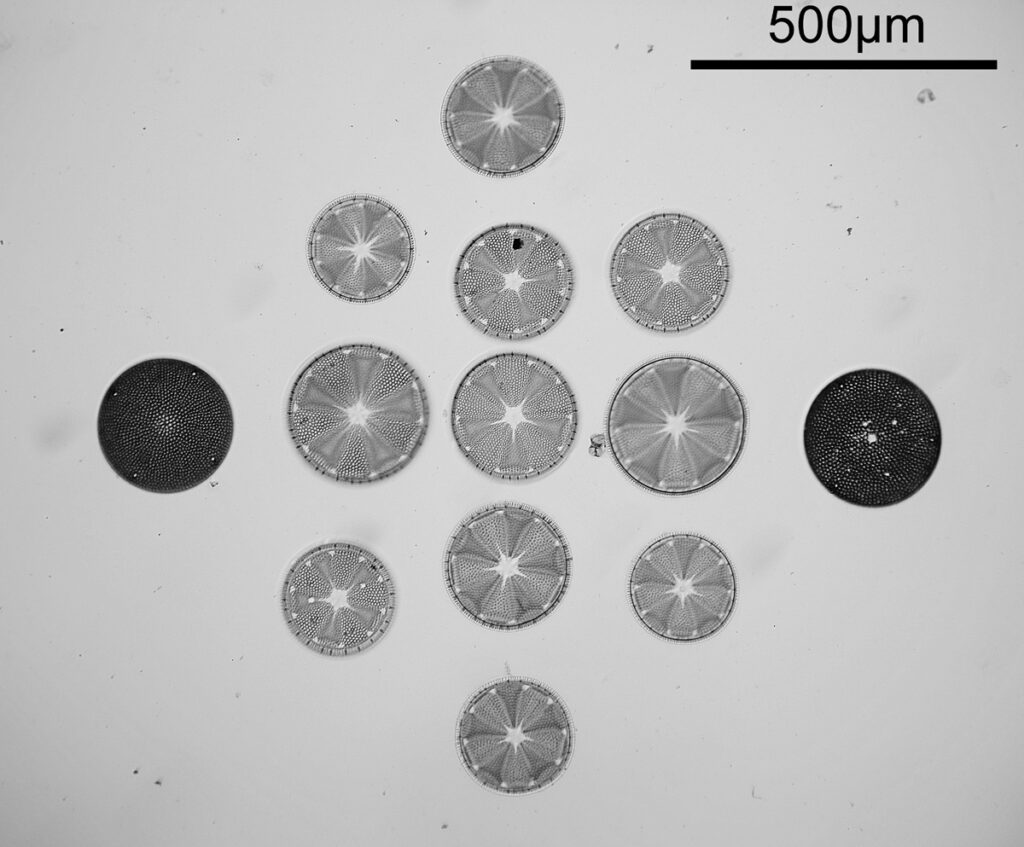
Next up is a Navicula firma slide. This has multiple examples of the diatom and was again imaged using oblique light from below, and with the 20x Splan objective.
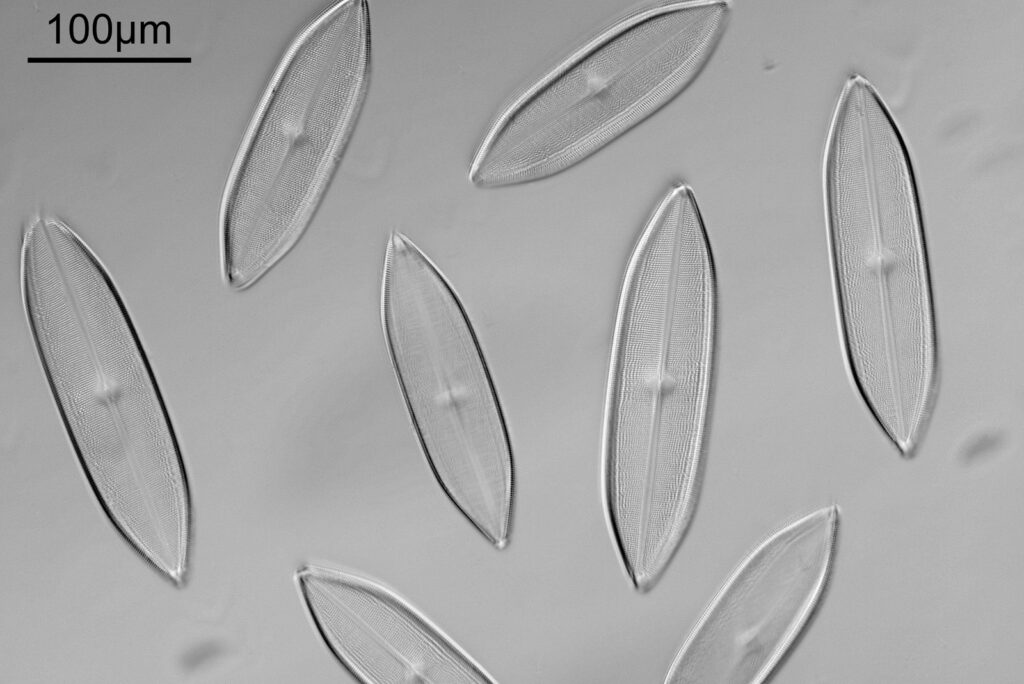
The diatoms are quite large but show some lovely features and will be well worth coming back to at a later date to look at in more detail. Here’s the slide.

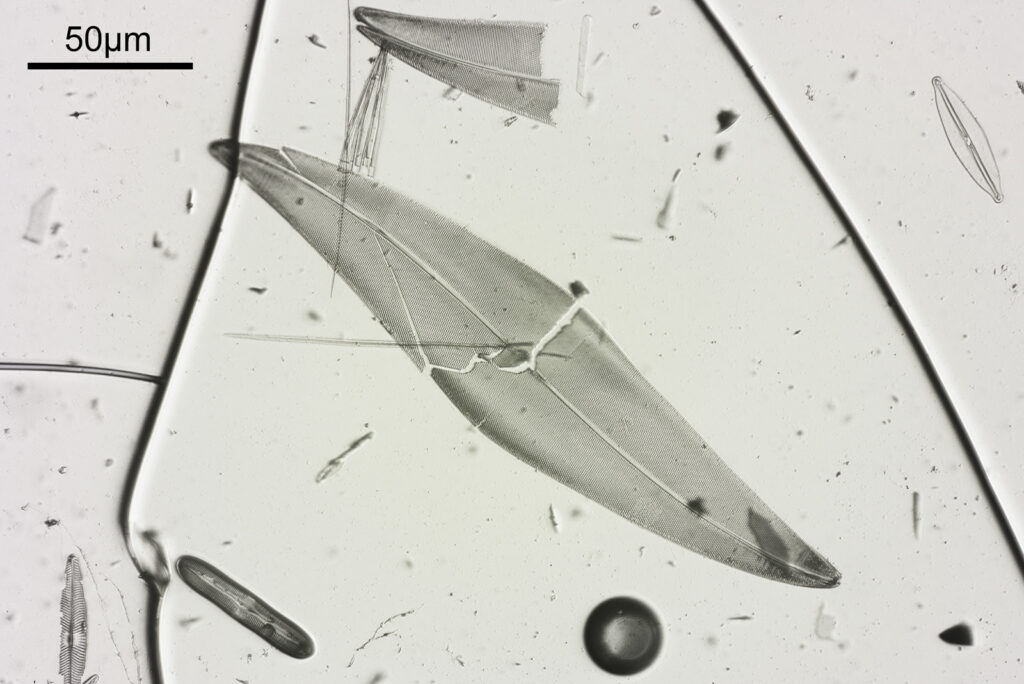
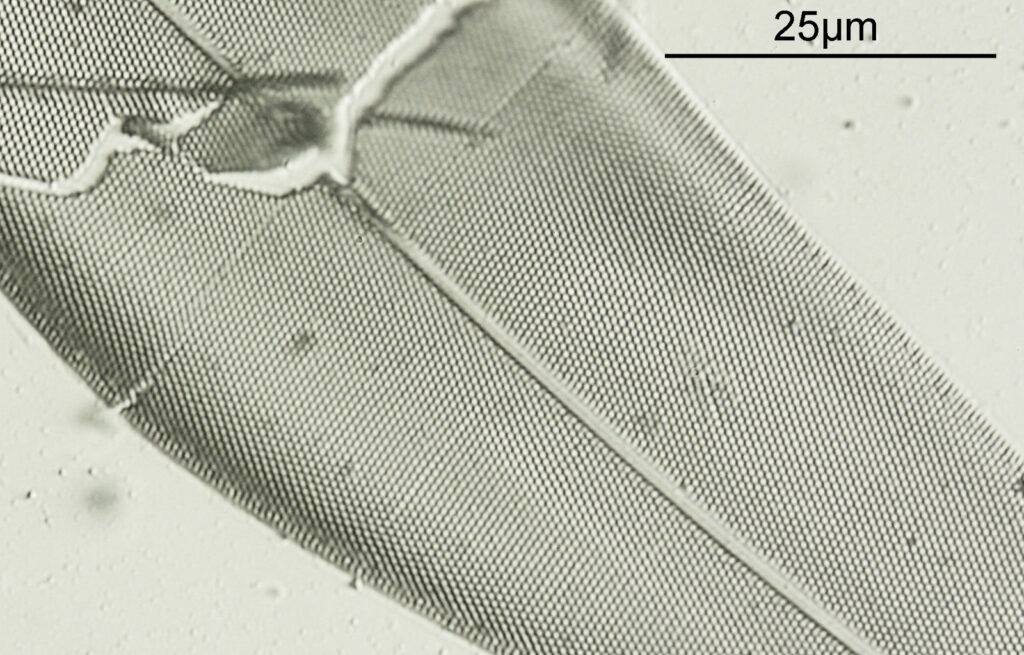
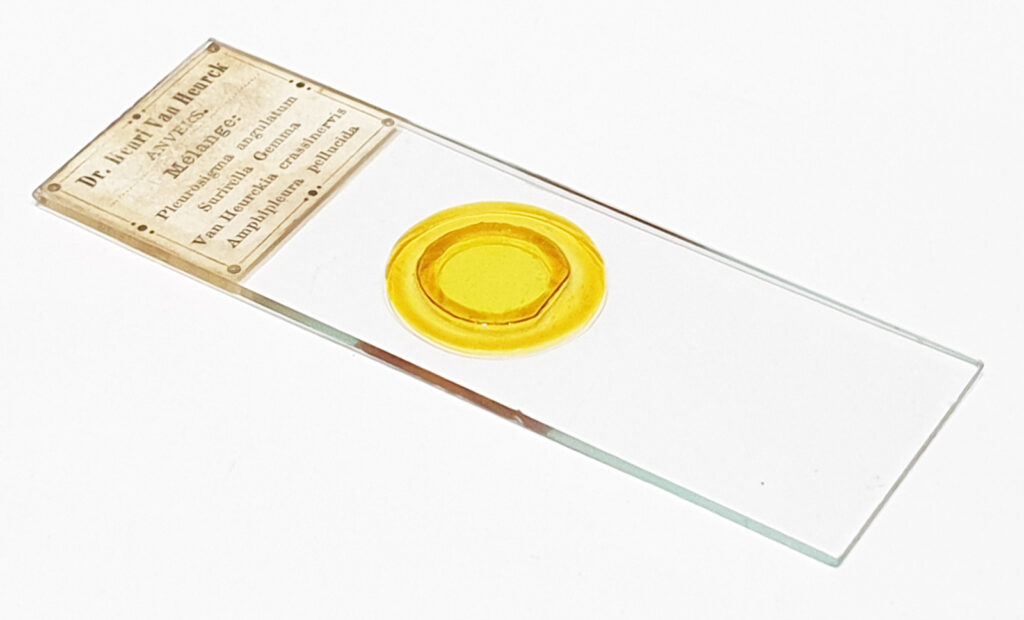
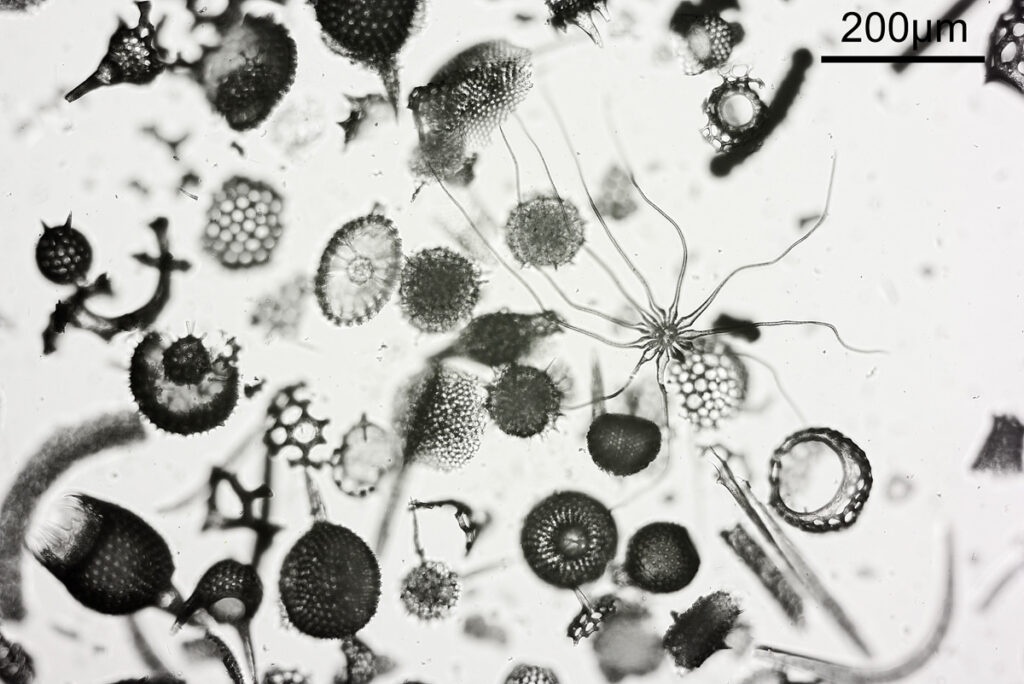
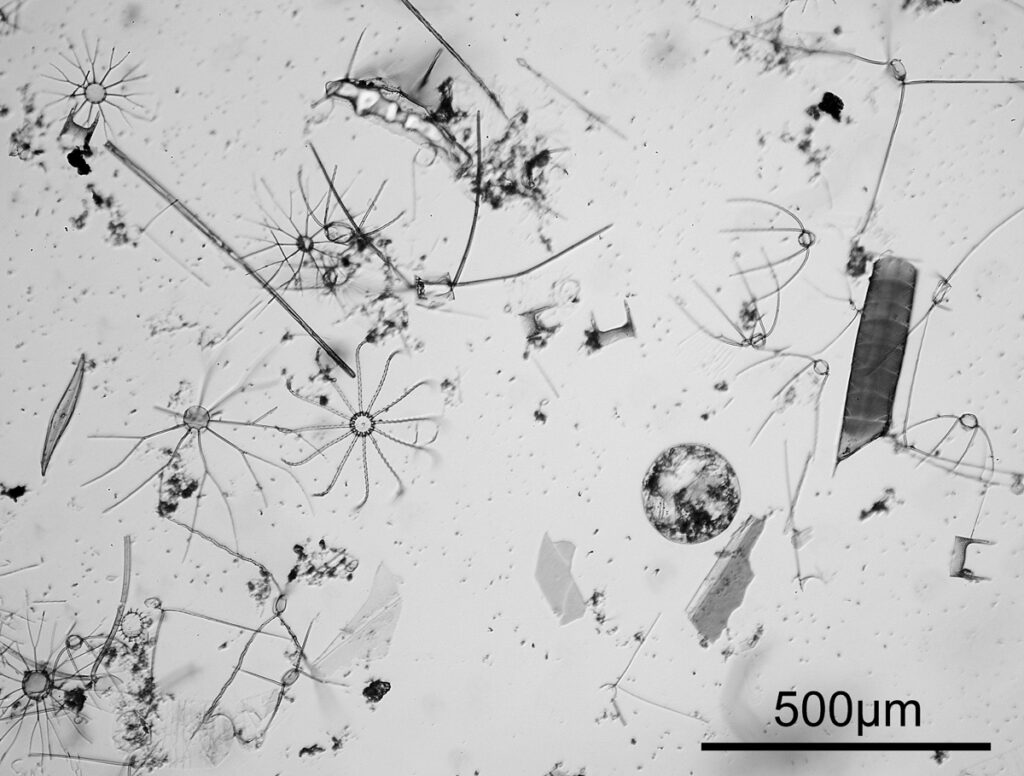
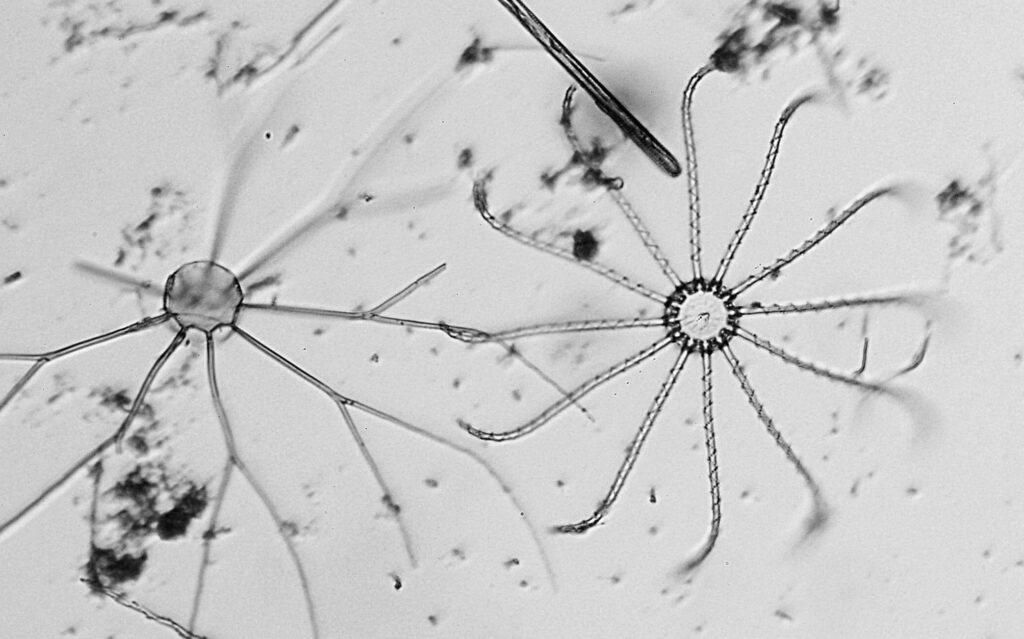
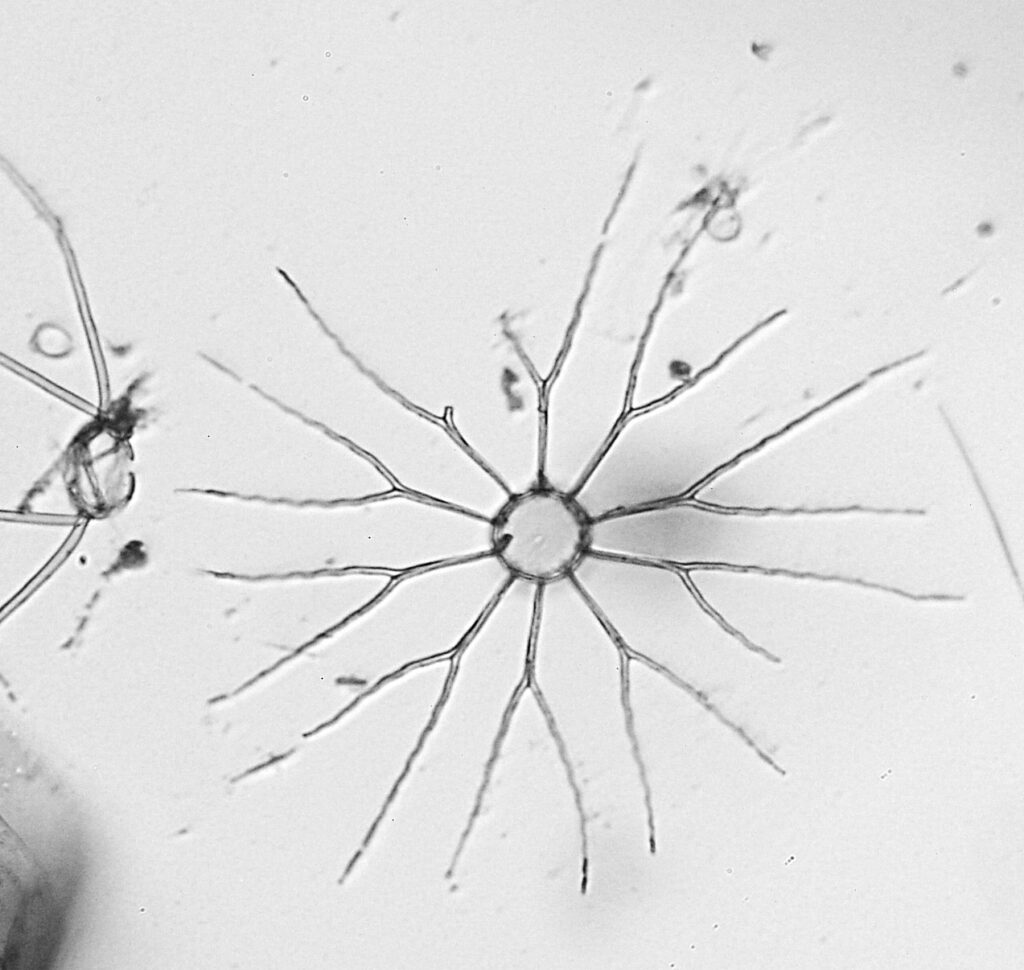
Next is a diatom and spicule strew made by Eduard Thum (one of my favourite slide makers). This was also imaged using oblique lighting from below and with the 20x Splan objective.
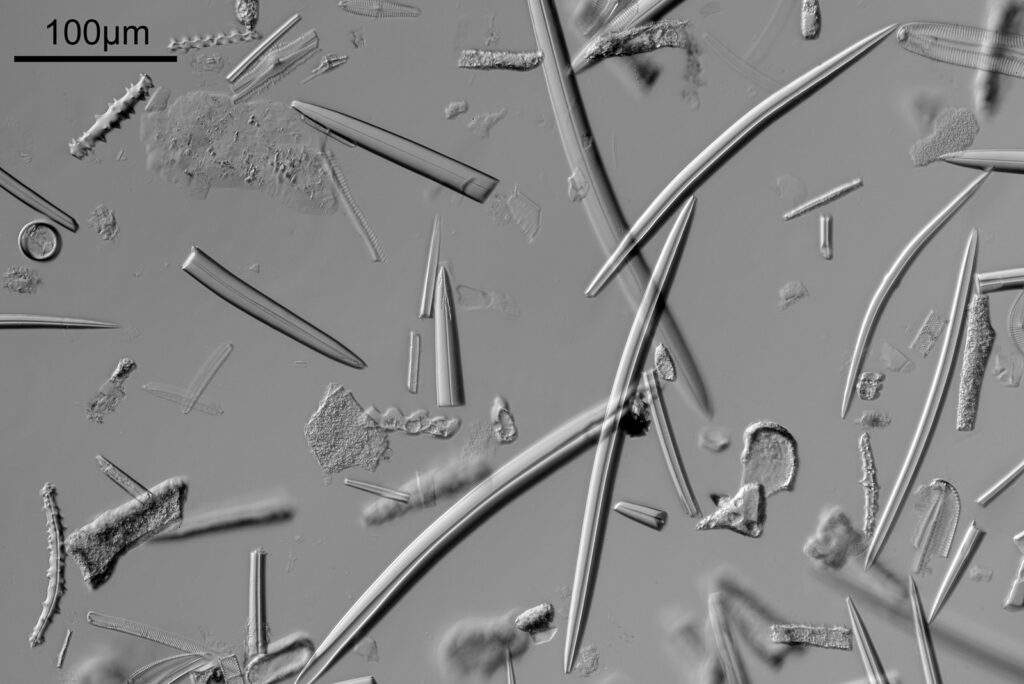
This slide is a strew so contains a wide selection of material to image. It seemed to lend itself very well to the oblique lighting, creating a great 3D effect. Here’s the slide.
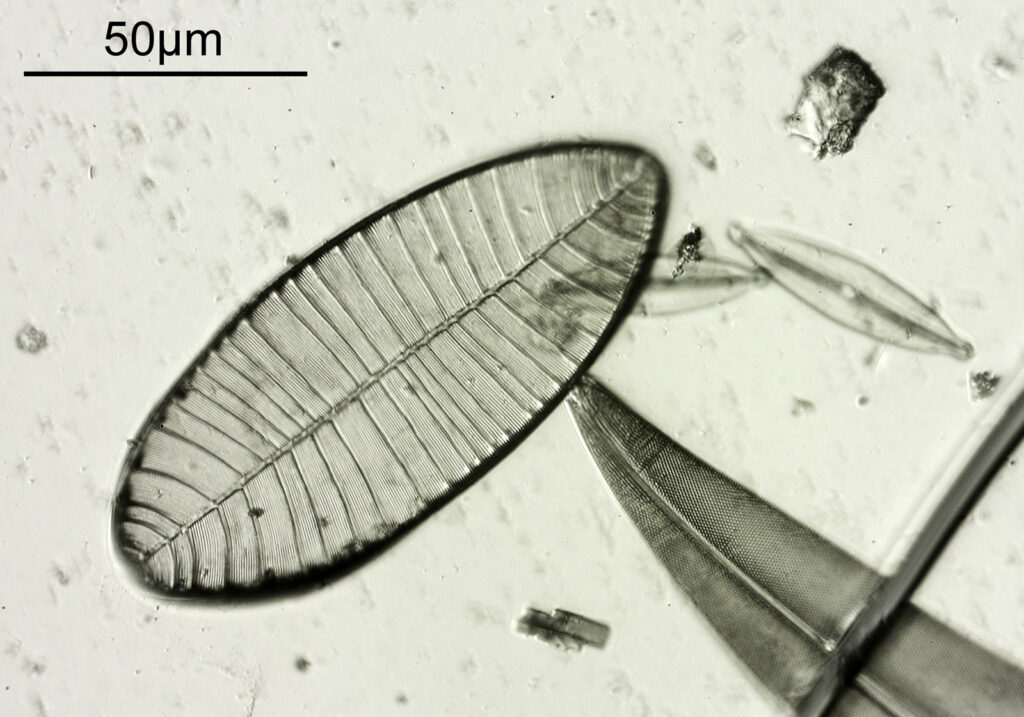
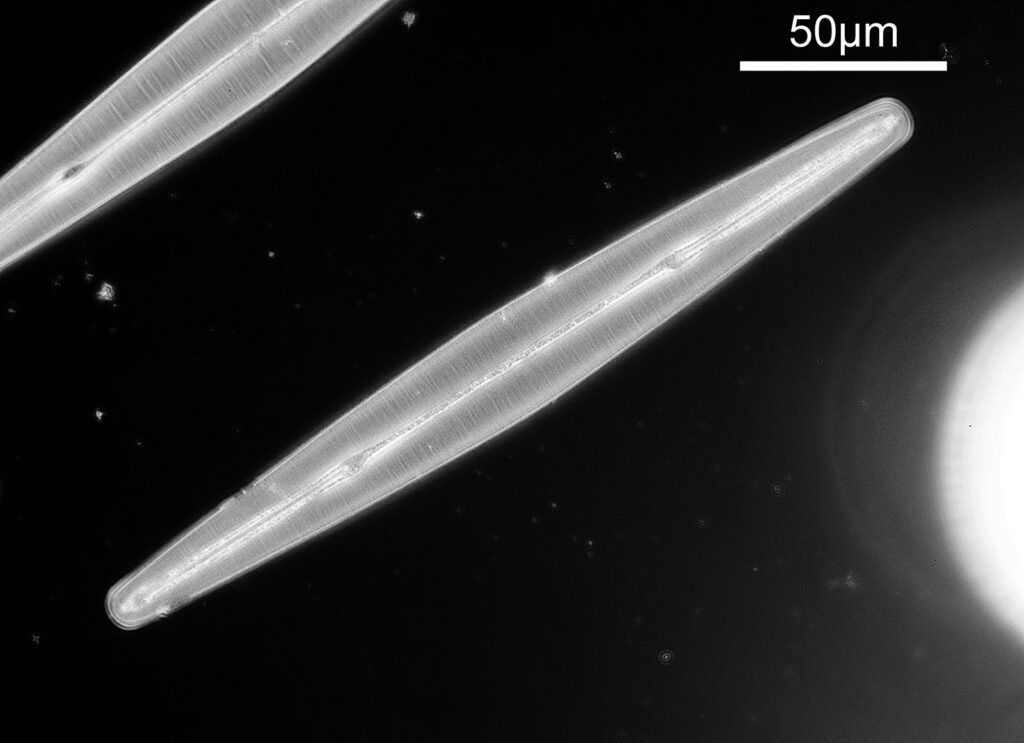
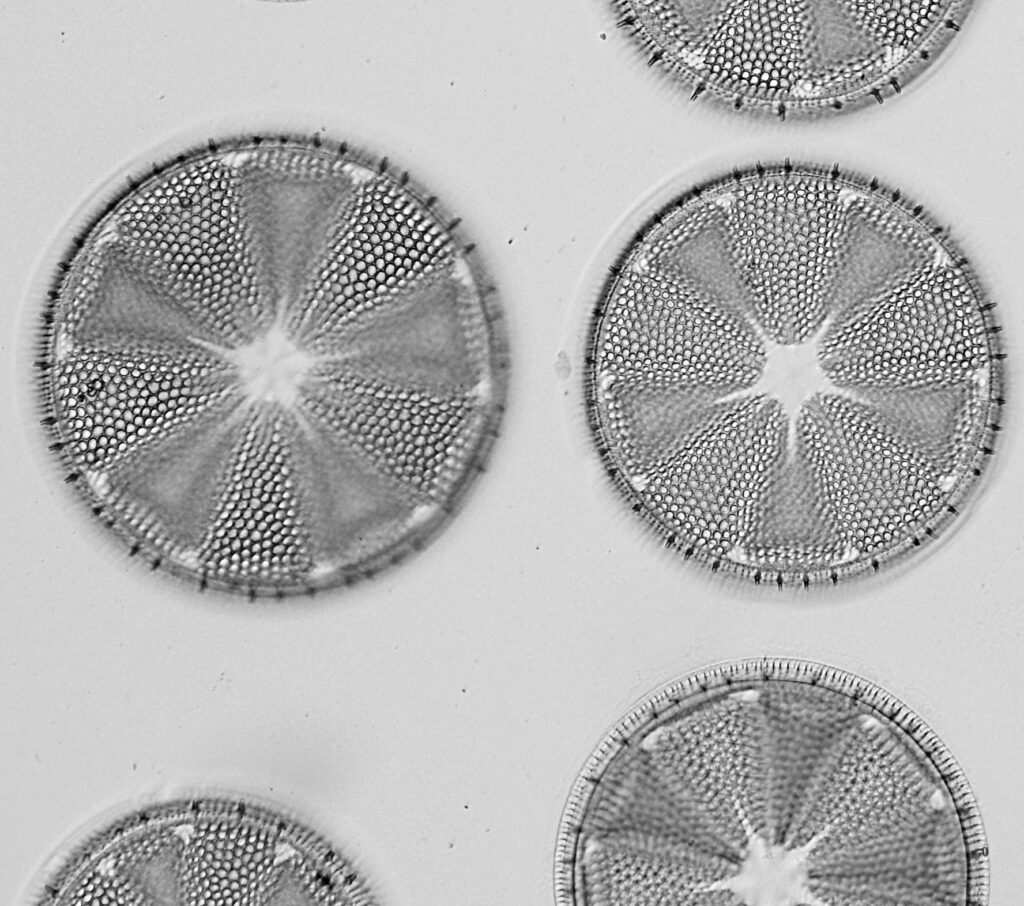
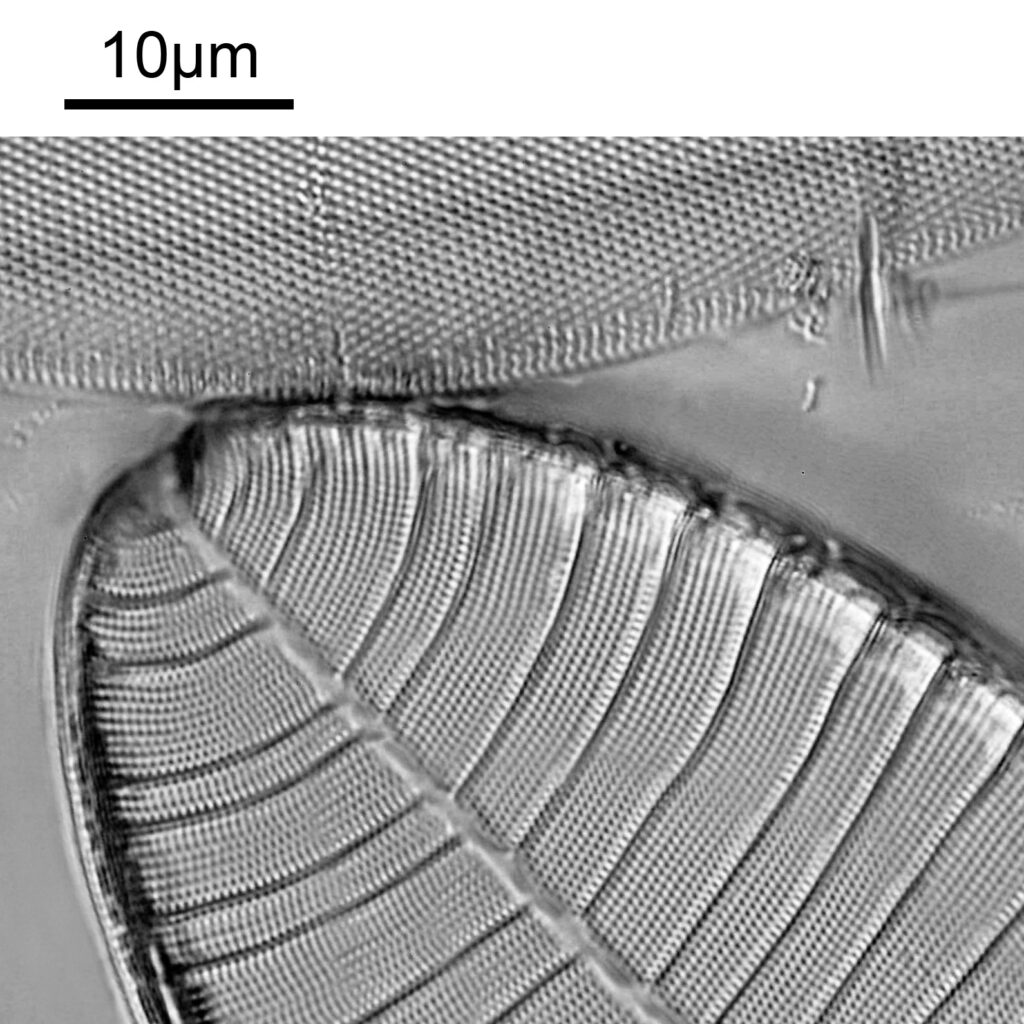
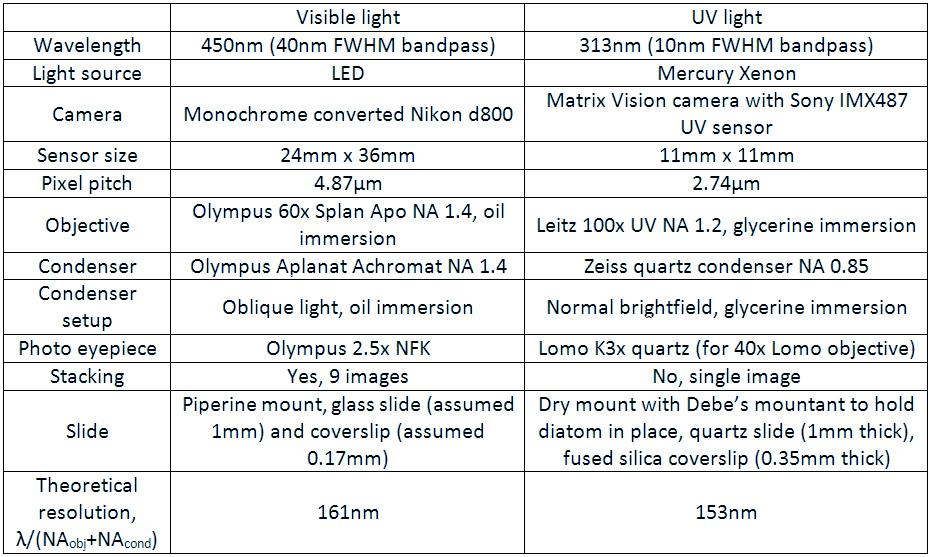
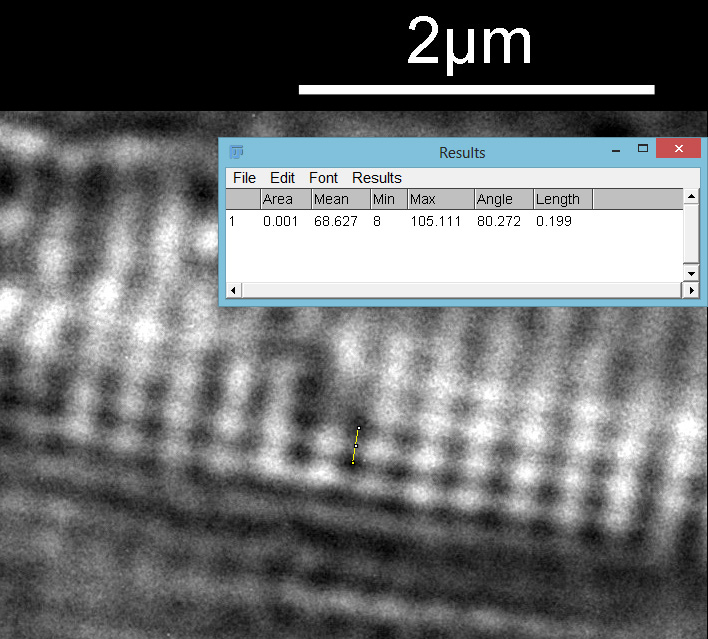
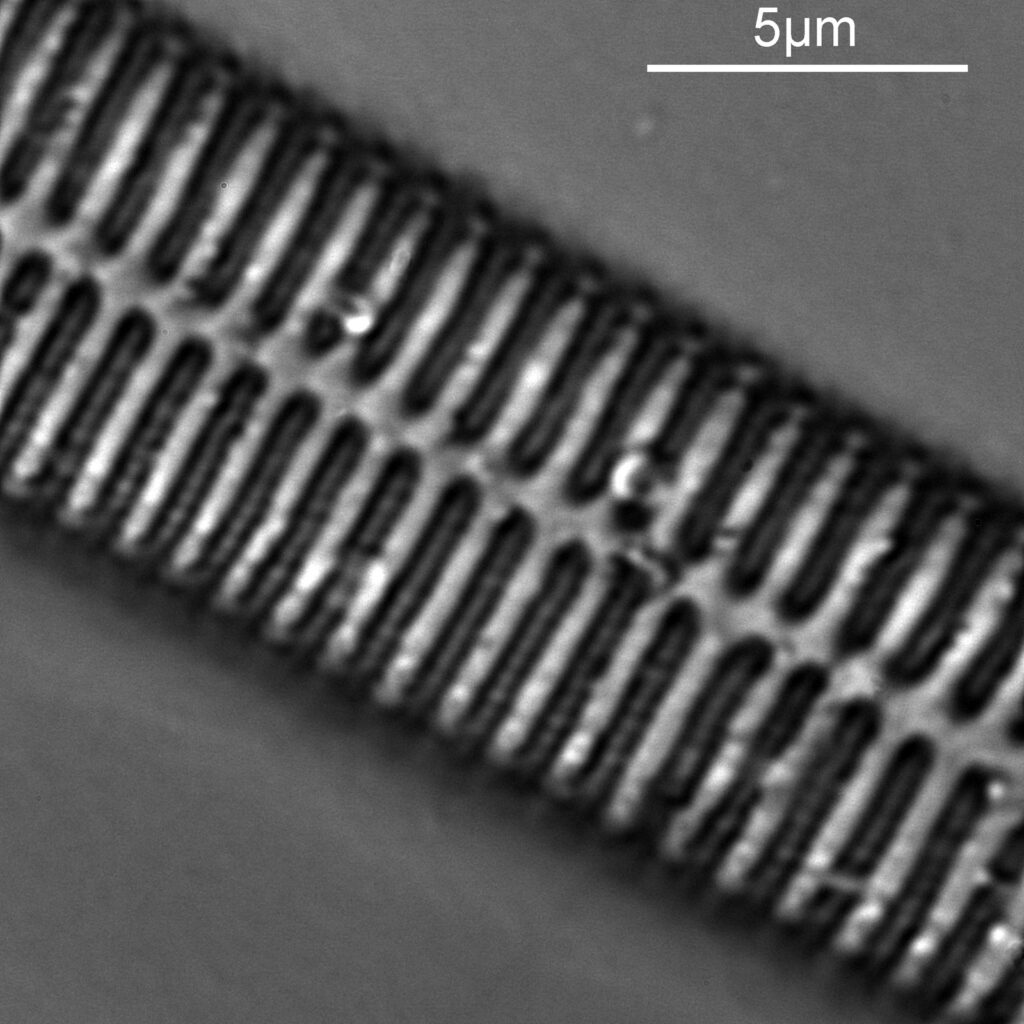
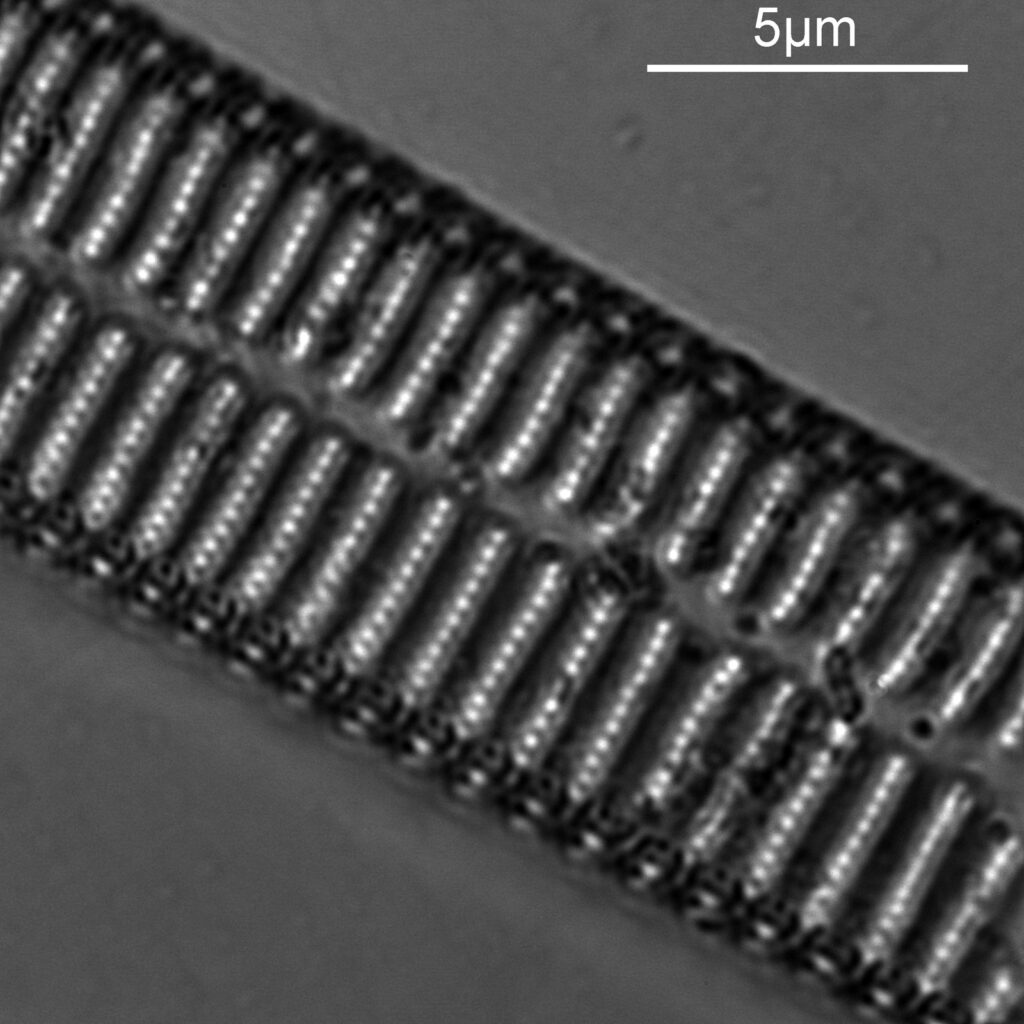
The final one for today is a Niztschia species slide by A.C. Cole. I spent a bit of time with this one. First a couple of images done with the 20x Splan objective.

Both the images above where taken with the same condenser with light coming from below – an Olympus Aplanat Achromat. In the first image the lower part of the condenser was moved to one side. A small movement to the side produces oblique lighting and nice shadows, however a larger movement (or closing down the iris further) as done here, produces a dark field effect with a black background. This can be very useful for highlighting features in the diatoms. In the second image the lower part of the condenser was returned to the middle, and normal bright field imaging was done. The resultant image is relatively low contrast, and it is harder to see some of the features of the diatoms. How you light the sample has a huge impact on how it appears.
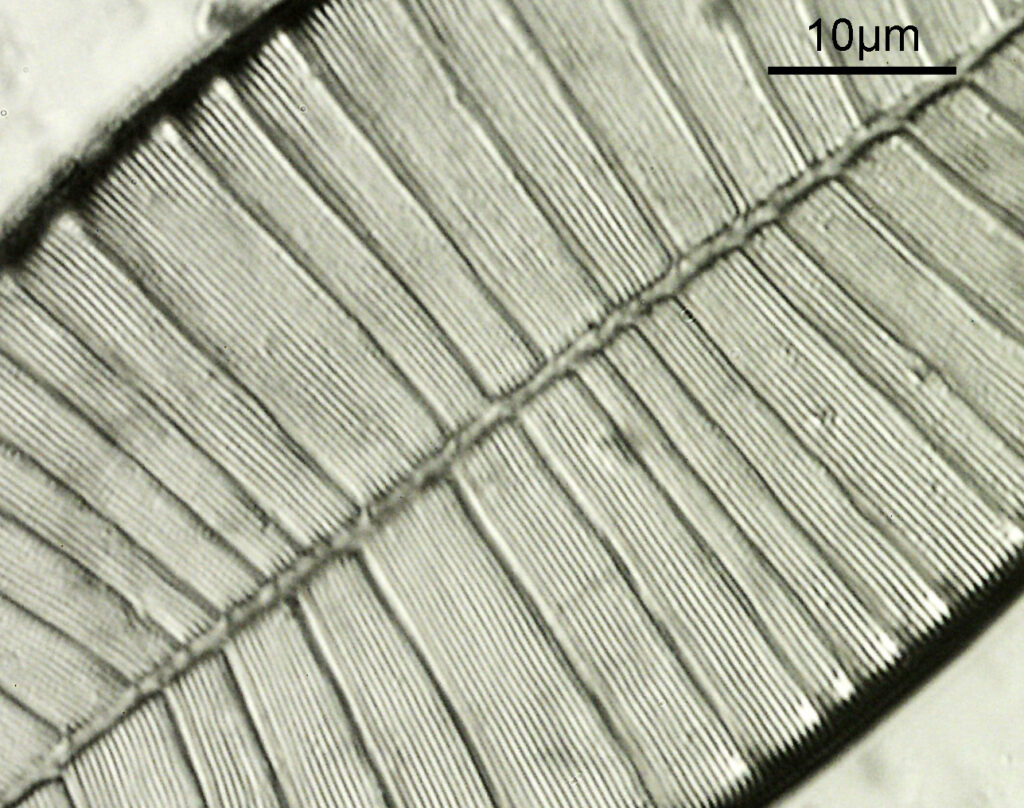
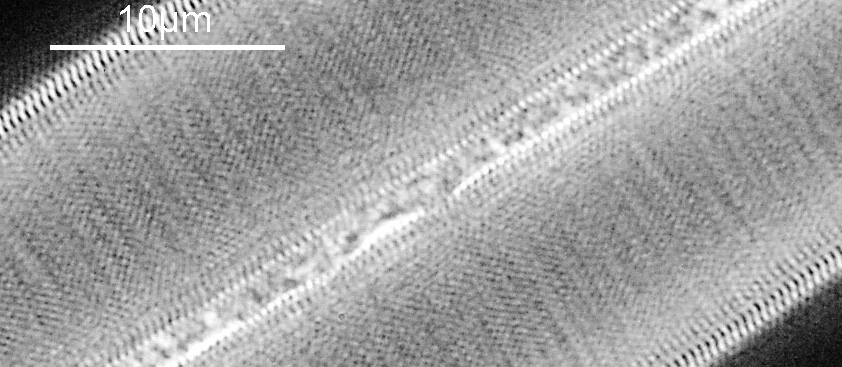
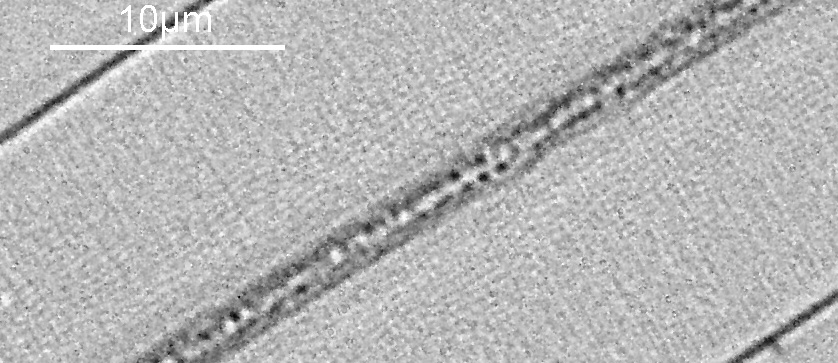
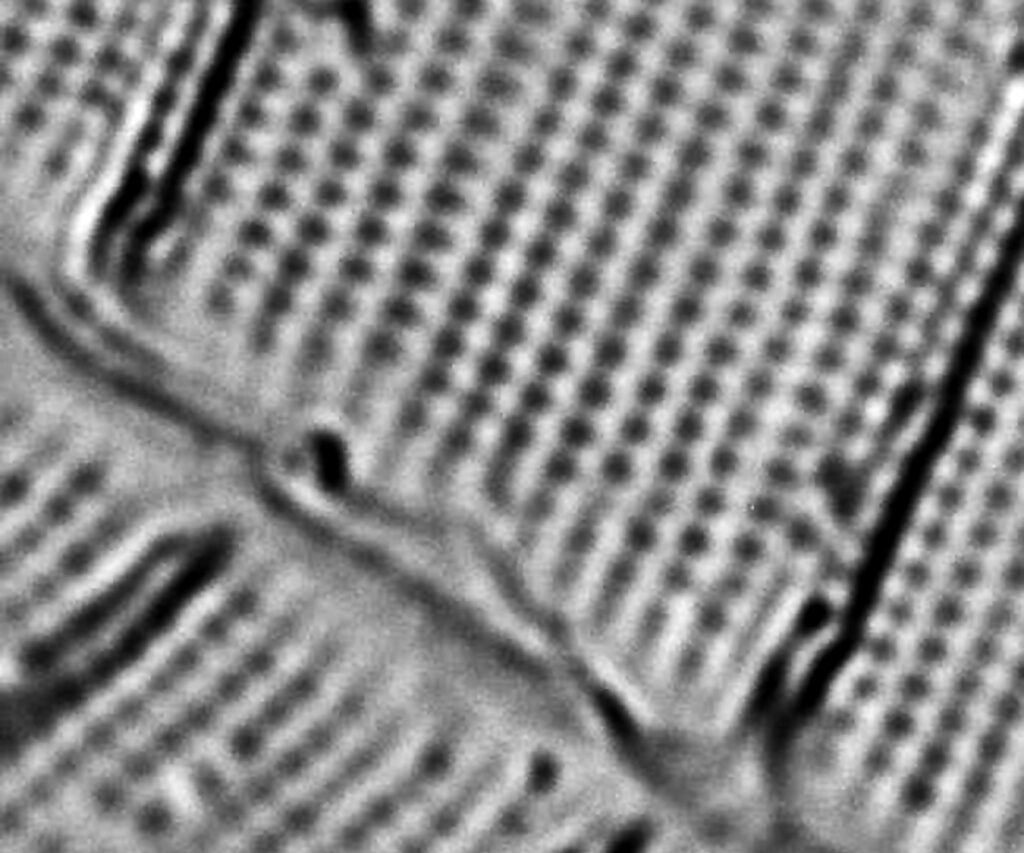
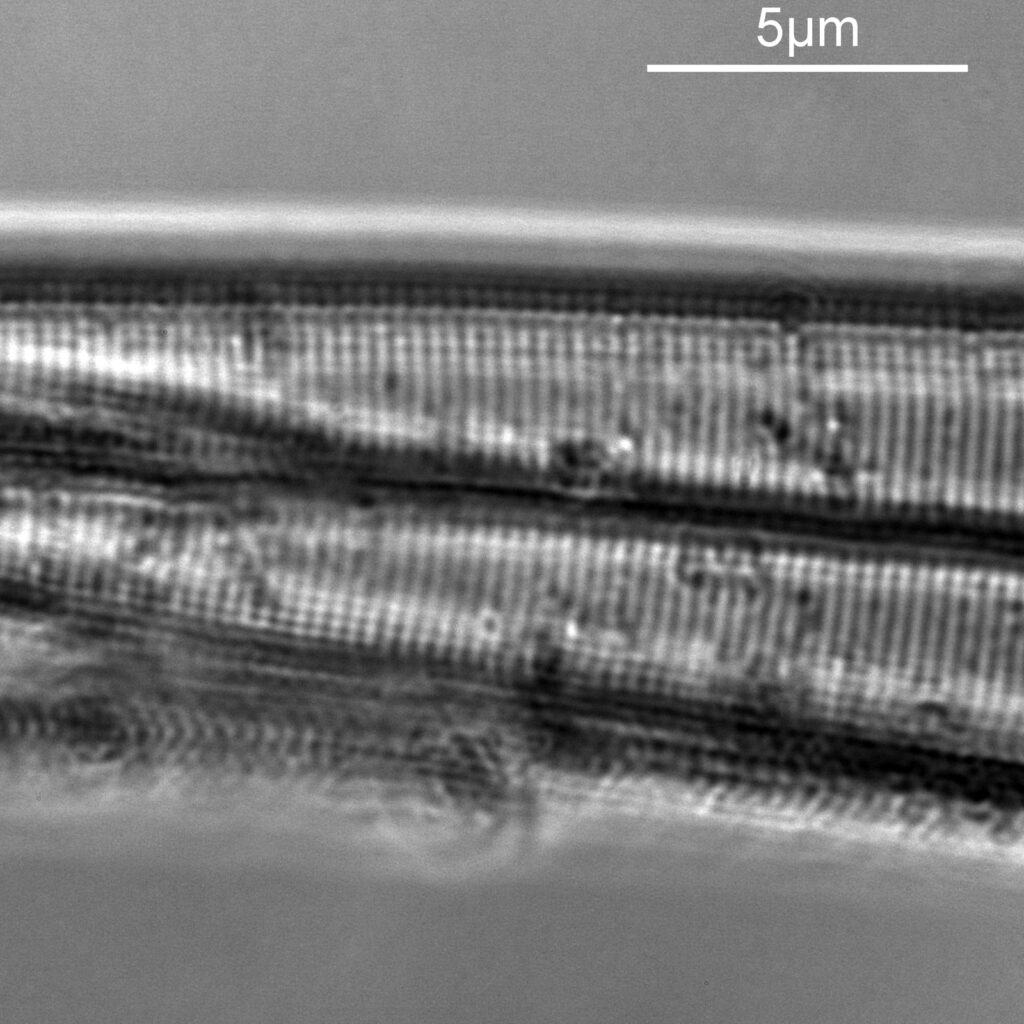
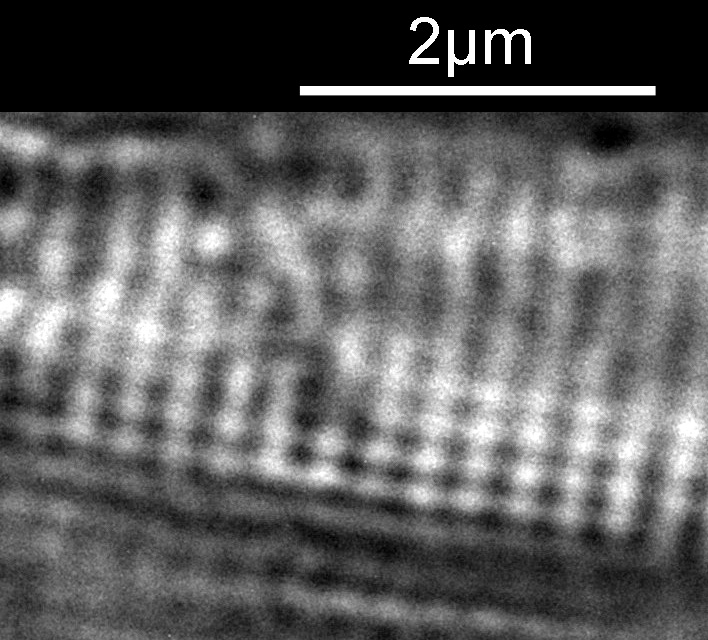
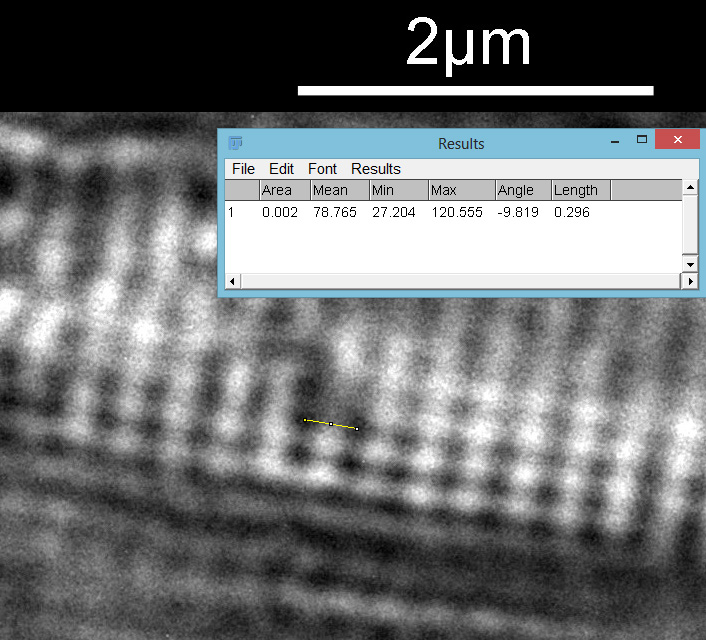
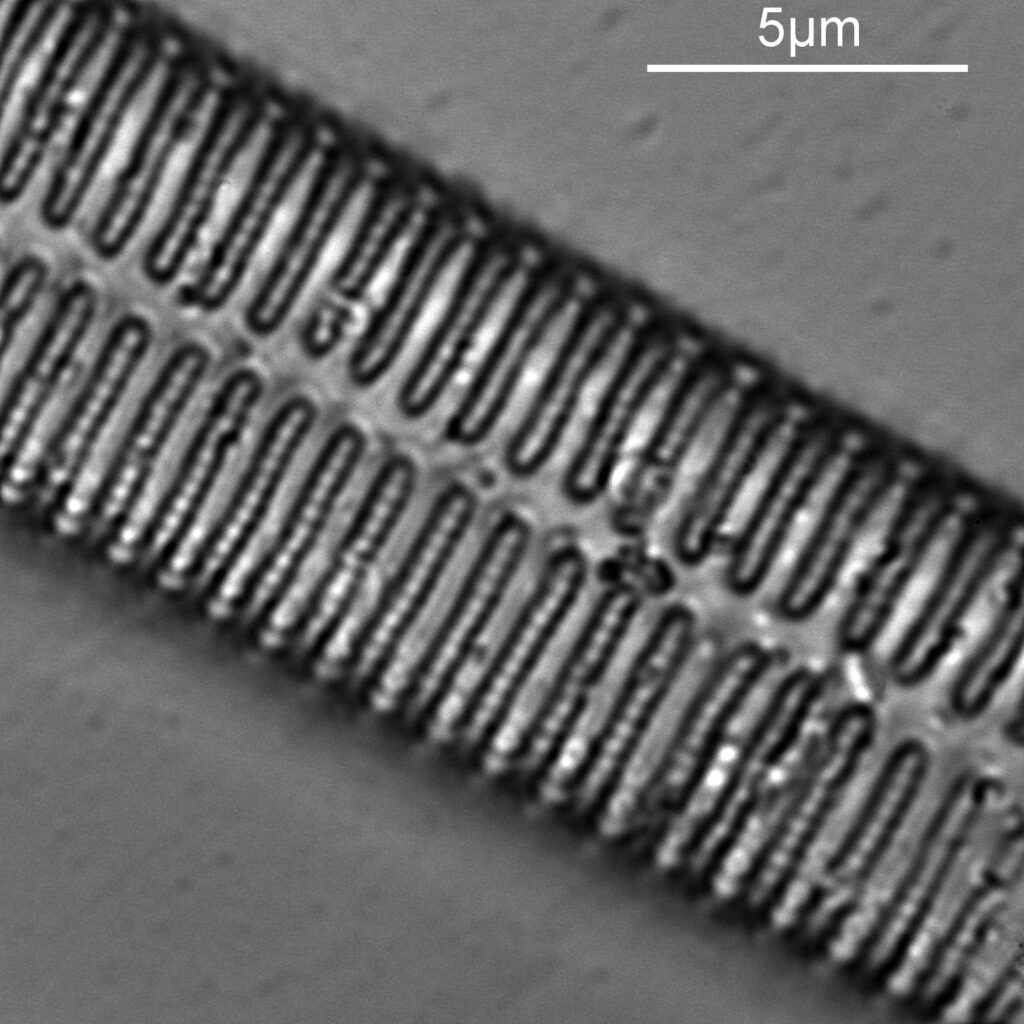
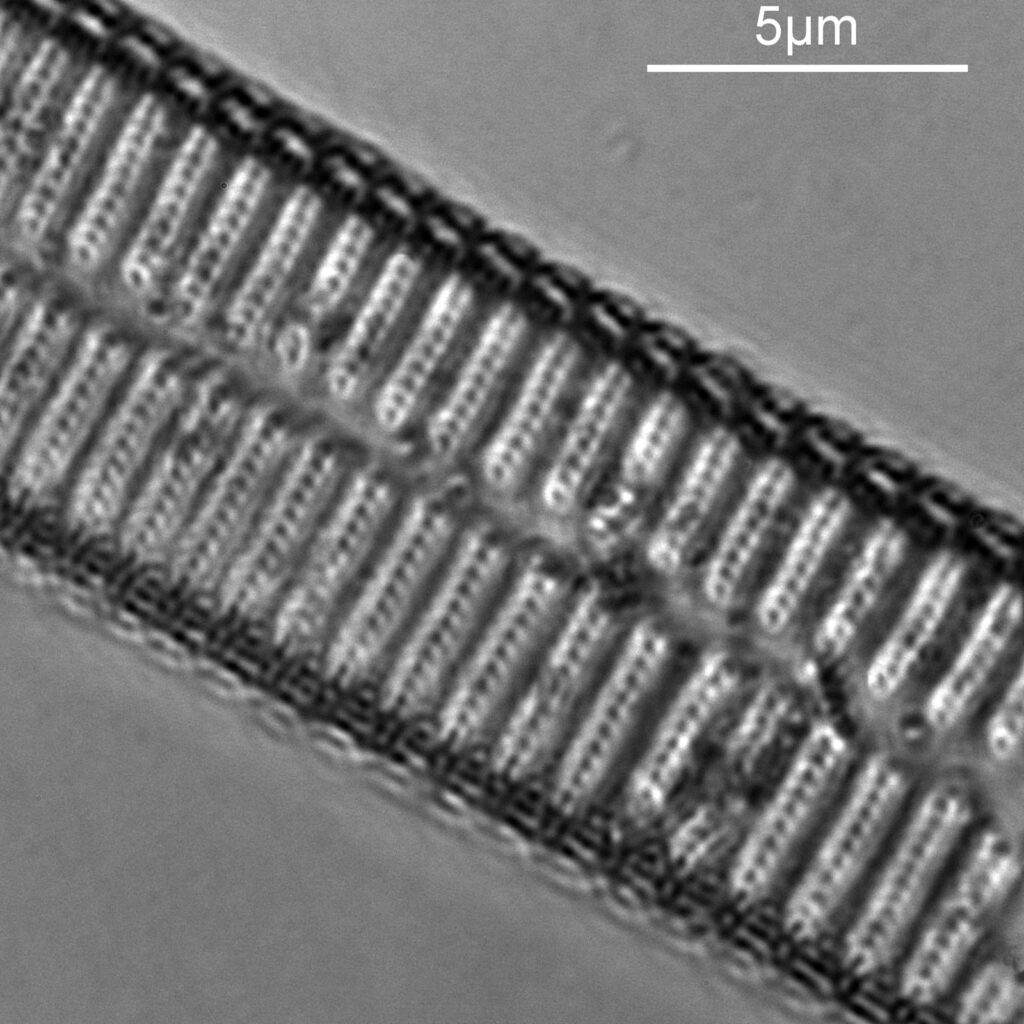
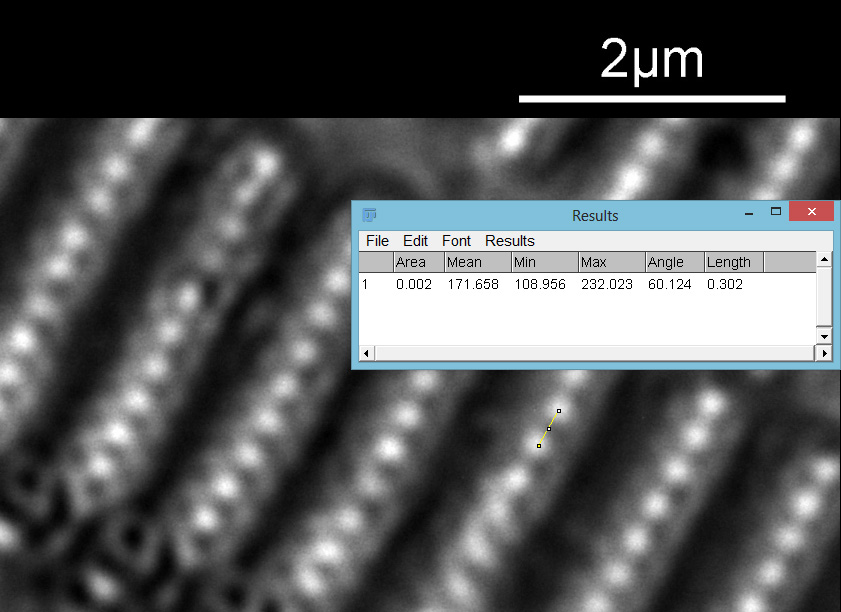
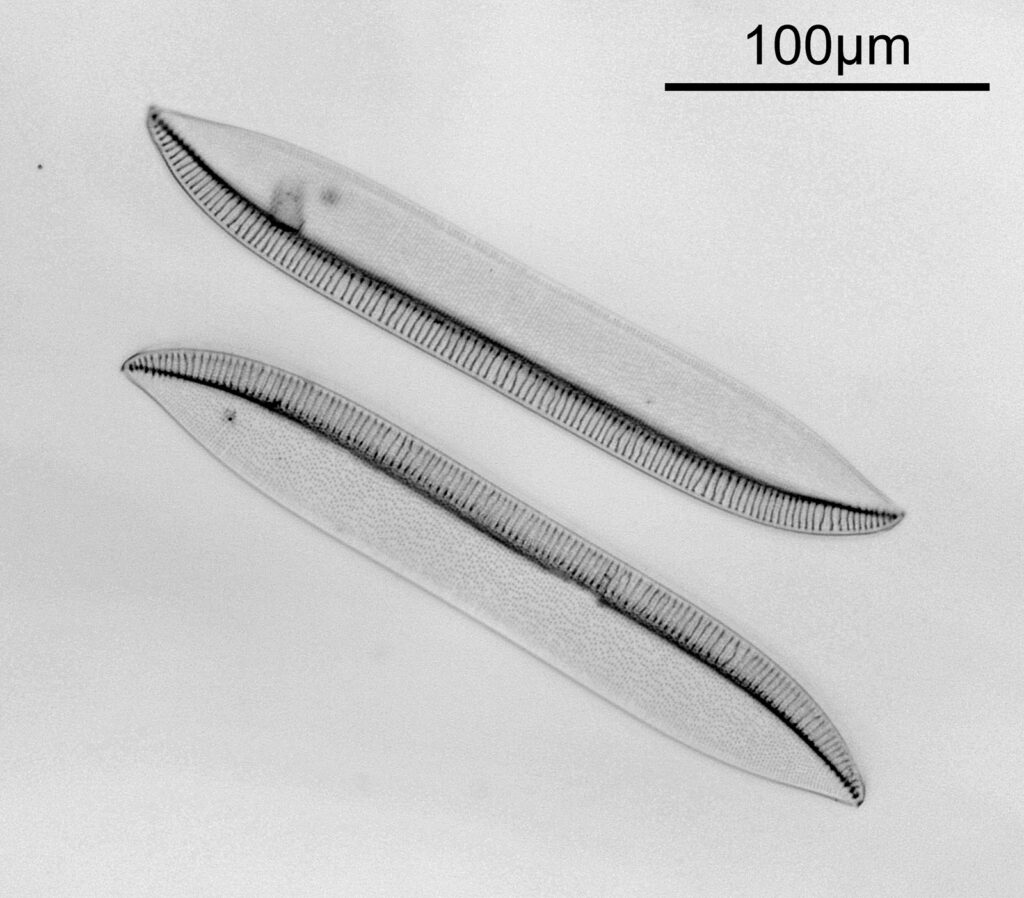
Next I imaged the same slide with a 40x Olympus Dplan Apo UV NA 0.85 objective. This has a coverslip thickness correction collar, and as the mounting of the sample had suffered over the years, the coverslip had tipped up slightly on one side. So I adjusted the correction collar to it’s max thickness (about 0.22mm). I also used 450nm filtered light to try and squeeze a bit more resolution out, and I set up the condenser for slightly oblique illumination. I did a stack of 7 images in Zerene stacker. This was the final image.

The higher resolution from using a higher NA objective and 450nm light is evident. The keen eyed amongst you will also have spotted the diatoms have been rotated by 180 degree from the images with the 20x objective. I took the slide off during imaging and must have put it back on the other way around when I returned it.
Stacking diatom images can be a challenge, as when the focus is changed different parts of the structure not only come in and out of focus, but look different as a result of the way light passes through the diatom structure. This can be seen in the video clip below, where I moved the stage upwards as the video clip was captured.
Here’s the slide.

This is an interesting slide, with the label on the back, so effectively the slide needs to be turned upside down to image (in the photo above the sample and coverslip are on the underside of the slide).
There are always plenty of diatom slides for sale on places like eBay, and while some go for high sums of money, others can be had for a few pounds. Some of the historic microscopists were capable of producing fantastic slides and as such these are a great way to get some beautiful slides to image. With imaging lighting is key, and changing the lighting setup can have a huge effect on the final image. As always if you have made it this far thanks for reading, and if you’d like to know more about my work I can be reached here.