A long time ago I did a PhD in surface chemistry at the University of Durham. My professor (Prof Jas Pal Badyal), is still there and still doing great research. Some of my equipment is still in the lab, and I was lucky enough to visit there earlier this year and see some of the work the team are doing. During my PhD, some experiments were done which produced results which I never fully answered. However with my new interest in microscopy it has helped me look at my PhD again with different eyes, and cast light on some of my observations from over 25 years ago. Today I’d like to share these with you.
The first was purely a few nice images from a microscope slide I bought. During my PhD I worked with precious metal compounds. I’d dissolve them up in different solvents, spin coat them on to different substrates and then hit them with a cold gas plasma to reduce them down to metal layers. The goal of this was to find new ways to make catalysts on temperature sensitive substrates. At the time, I’d often get nice complex crystal structures and a few of these even made it into my PhD as rather poor quality photos. The microscope slide I got recently was of potassium platinum cyanide (a platinum compound) on glass. Under crossed polarized light, which is often used for the imaging of crystals, this is what it looks like.
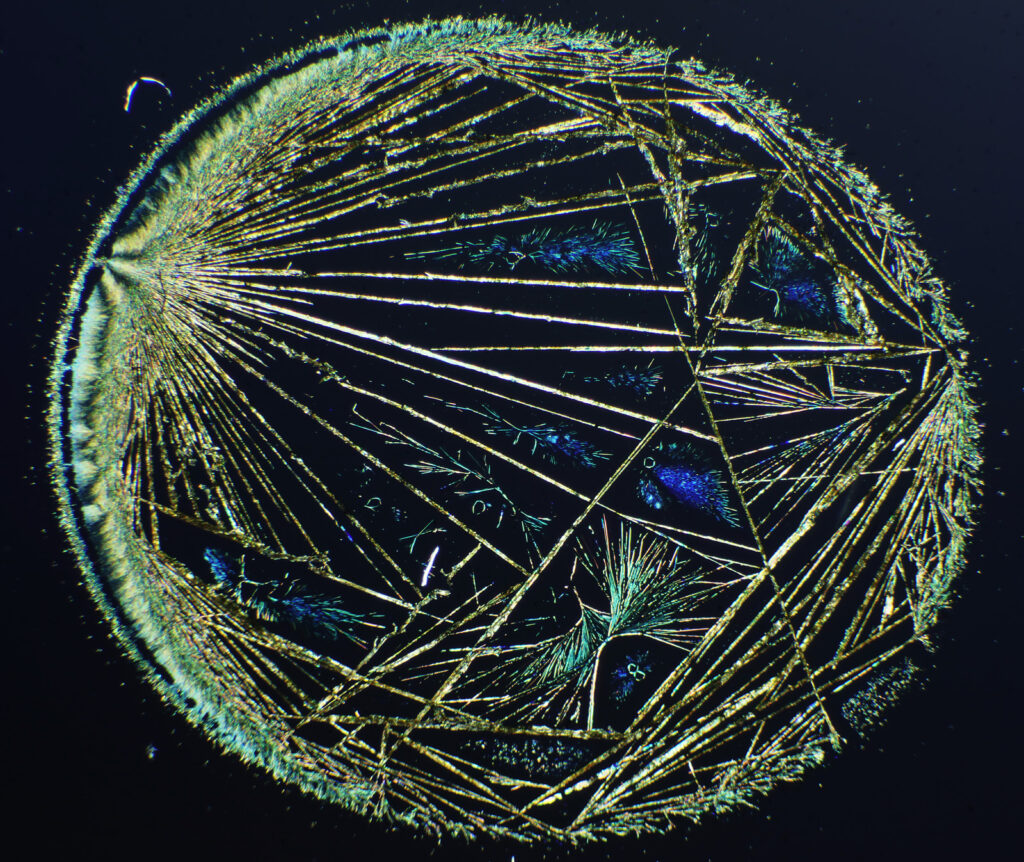
The image above was taken using an 1x Olympus SPlan FL1 objective, on my modified Olympus BHB microscope. The whole circle is about 6mm across. I removed the condenser and just used the field lens to cast light onto the sample given its size. Very often I’d get complex dendritic structures like this with silver and palladium compounds, and I struggled to photograph them with what I had to hand at the time.
Here’s some more images from this slide as it is so beautiful and complex. These were taken with a higher magnification objective (a Nikon 10x NA 0.5 Fluor). If only I’d had this microscope back in my PhD….
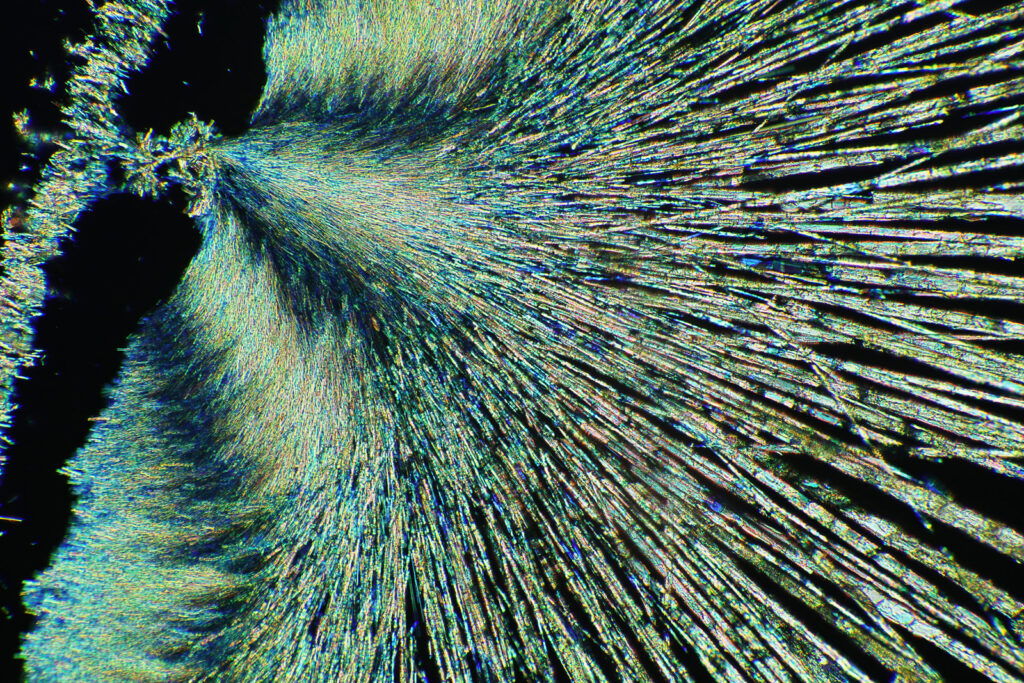
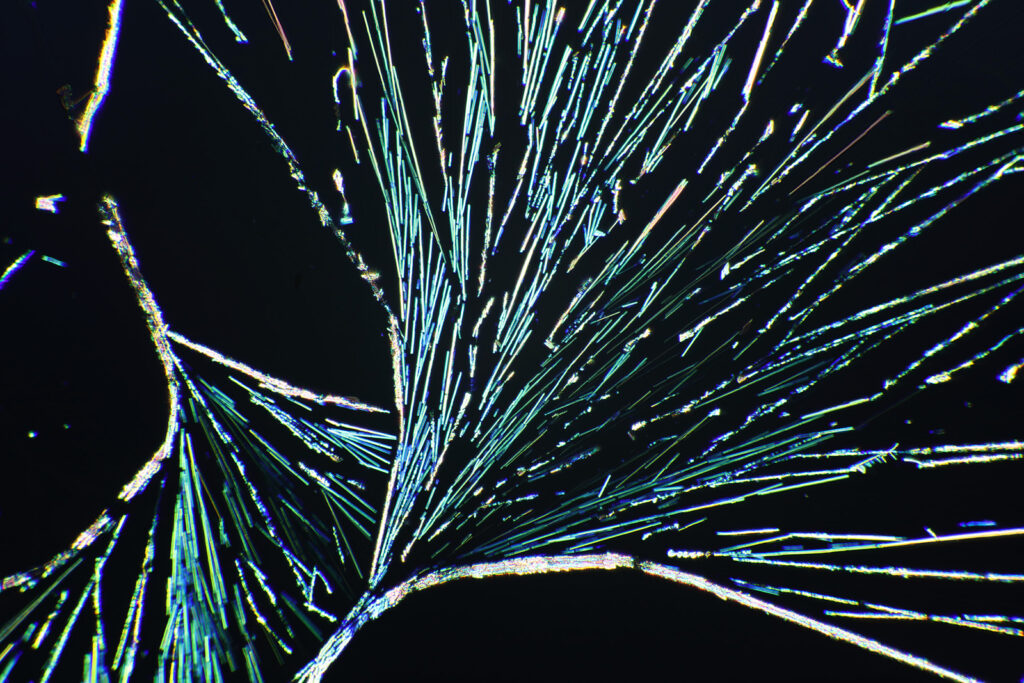
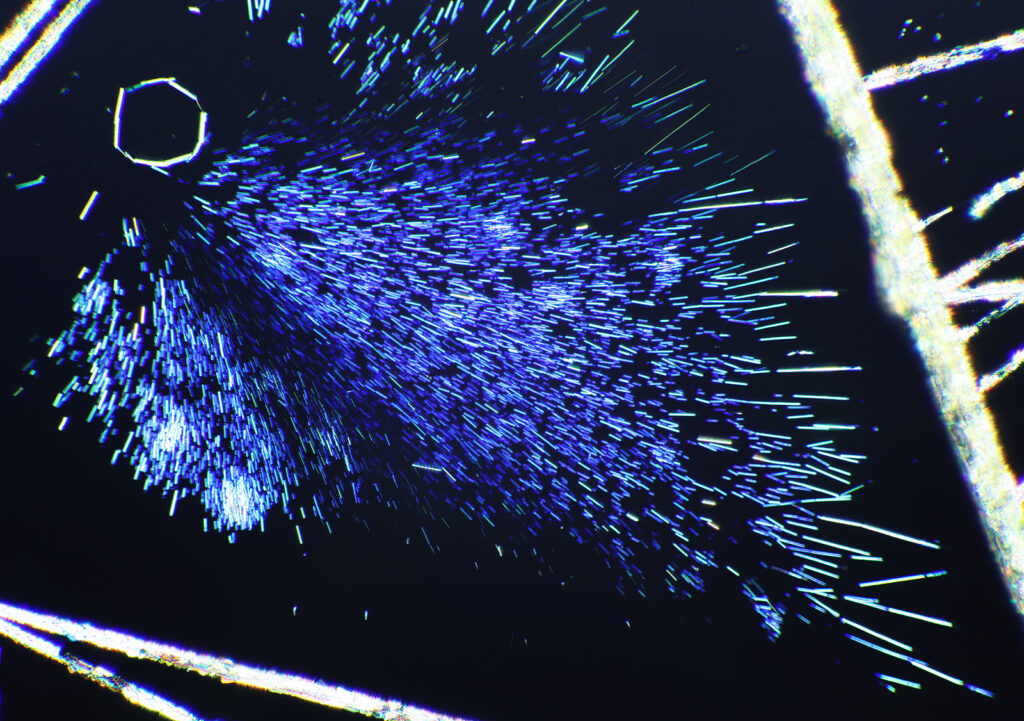

The second thing is a bit more of a technical one, and the purchase of a book gave me some answers I could have done with when I was back in the lab. This came about because of a chance observation one day. I used to deal with gold chloride (AuCl3) solutions. These were spin coated on to Nylon, and the result was the Nylon would slightly dissolve, and the gold salt and Nylon would mix together to form a thin layer. Normally I would then treat this with a hydrogen plasma to make a gold metal layer. To analyse the samples I used a range of different techniques, one of which was X-ray Photoelectron Spectroscopy (XPS) which involves using X-rays to irradiate a sample, kicking out electrons which are then collected and analysed. The energy of the electrons tells you about which elements are present. I noticed that with some of my gold chloride samples, if I analysed them before treating them with the hydrogen plasma, they would come out of the device looking different where the X-rays had hit the sample. They would be a different colour, often red or brown. Sometimes this sample would then change over the next day or so and become more ‘gold’ looking. Essentially, the X-rays were doing something to the sample. Here’s some of my original notes from my lab books from 1996 (and yes I still have my PhD lab books, and no my hand writing has not improved over the years). The little ‘letter box’ shaped things in the text are actually the coated Nylon samples from the experiment – I stuck them in the lab book where possible.



In the second image above, one of the samples looks to be transparent red, and this gives you an idea of what they looked like (this one never changed over time). When I originally did the work I suspected this effect was due to the X-rays breaking down the gold chloride molecule and forming nano gold clusters or colloids suspended in the Nylon polymer matrix. Depending on the precise nature of the X-ray treatment with some of these samples the gold clusters then migrated over time to form larger structures and eventually more coherent gold films. However this was a distraction from the actual work, as my main focus was plasma treatment, and I was never able to prove what was going on.
A few months back, I came across a book called “Colloids and the Ultramicroscope” by Zsigmondy, and translated by Alexander in 1909. This was for sale in the US, and being a bit of a nerd I bought it as ultramicroscopy was a technique I was interested in reading about as it was an approach to produce very high resolution images. Here’s the book.


It turned out that he had used this technique to look at gold colloids, and amazingly the book had some tables and colour plates of gold colloid solutions with different sized particles.
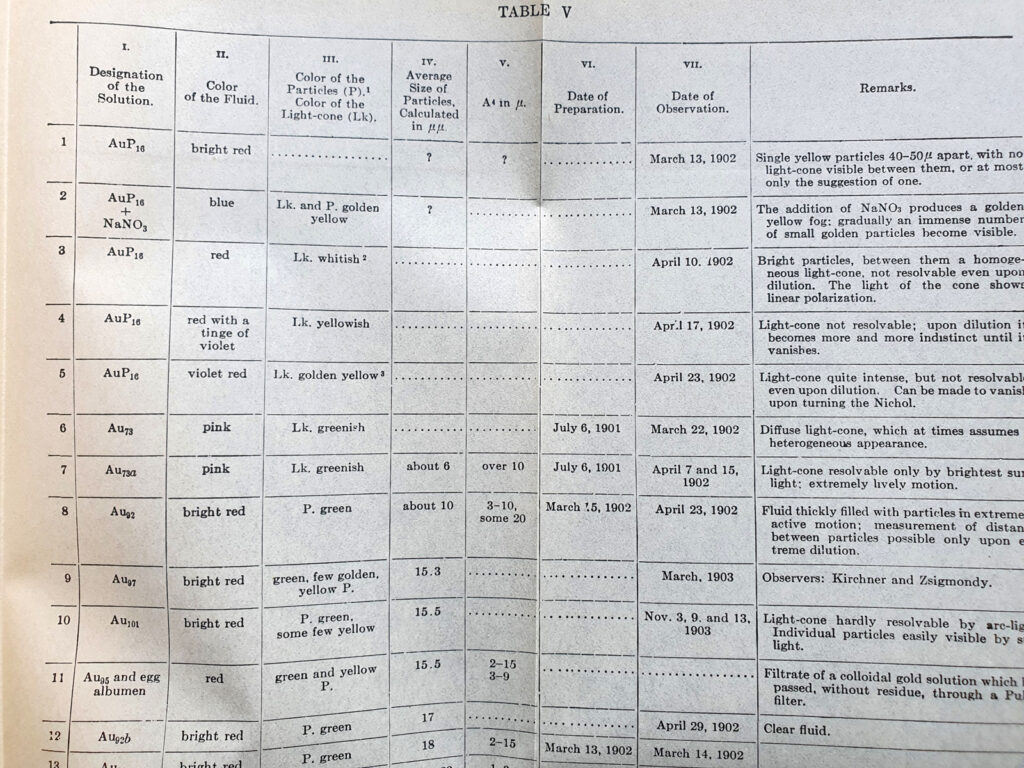
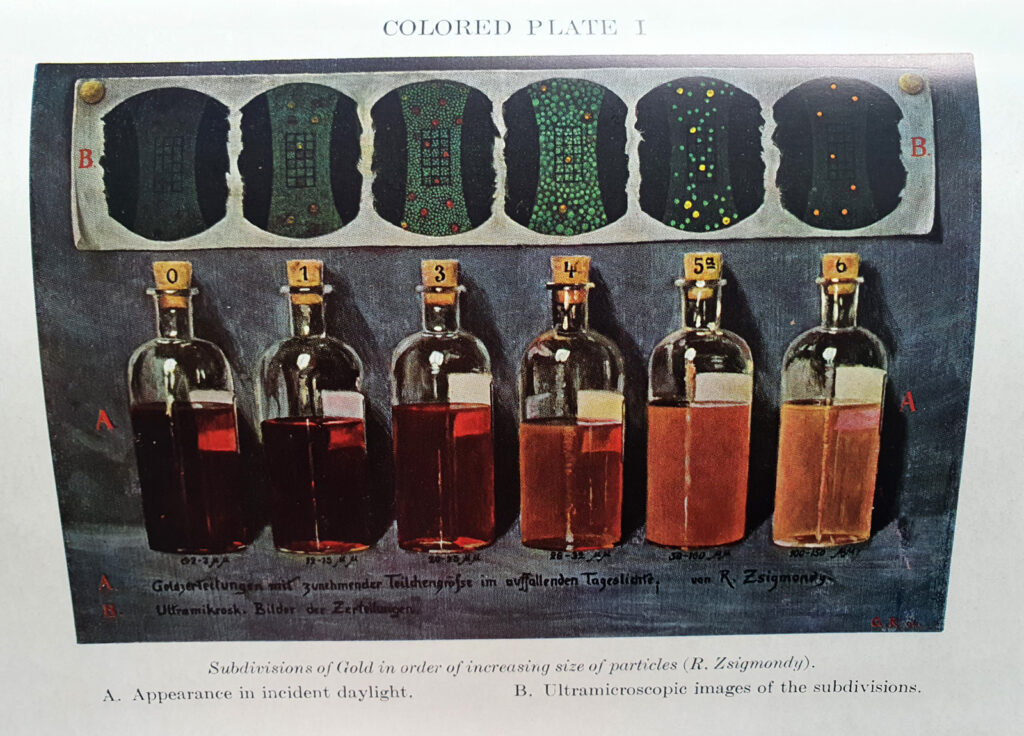
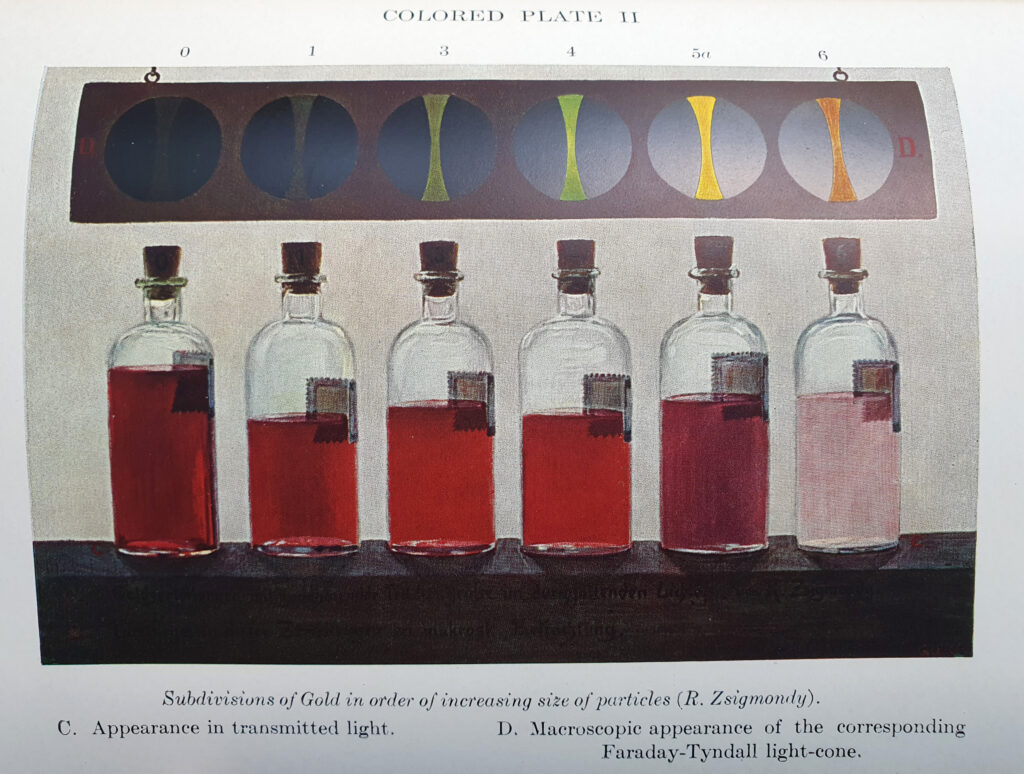
It does indeed look like small gold colloids have that distinct red colour which I observed – the smaller the particle the more intense the red.
Very often as scientists our experiments throw up questions which we cannot answer at the time. Unfortunately, in today’s deadline driven world, these are often seen as problems – things which slow us down and distract from the desired goal. But the key driver for a scientist is exploring and hopefully explaining the unknown. Sometimes this happens quickly, but as in this case it can take years, and inspiration often comes from unexpected sources.
As always, thank you for reading, and if you’d like to know more about the work I do, I can be reached here.
