Continuing with my microscopy journey, I thought I would try imaging an eye cross section to get a better understanding of the visual organ. After having missed out on a couple of old slides of eye sections, I ended up getting one from a seller on eBay (here described as Eye Entire VS). I’m fairly sure this a ‘mammal’ eye, not specifically a ‘human’ eye, but it’s interesting nevertheless. Here’s what it looked like.
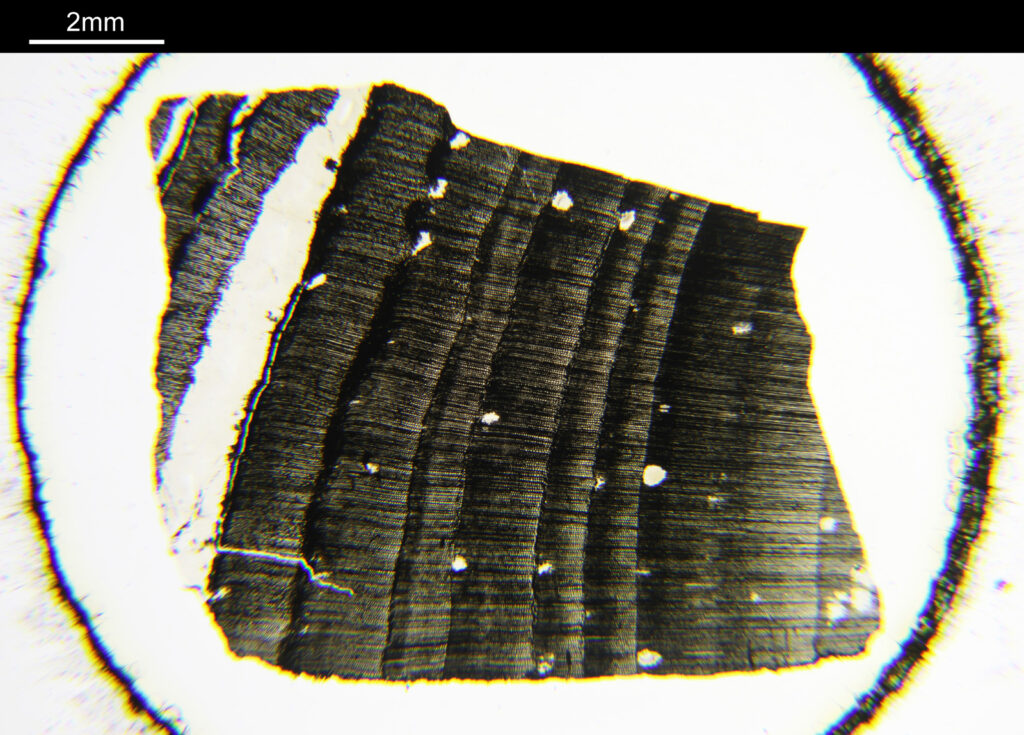
First an image of the whole specimen with a 1x objective.
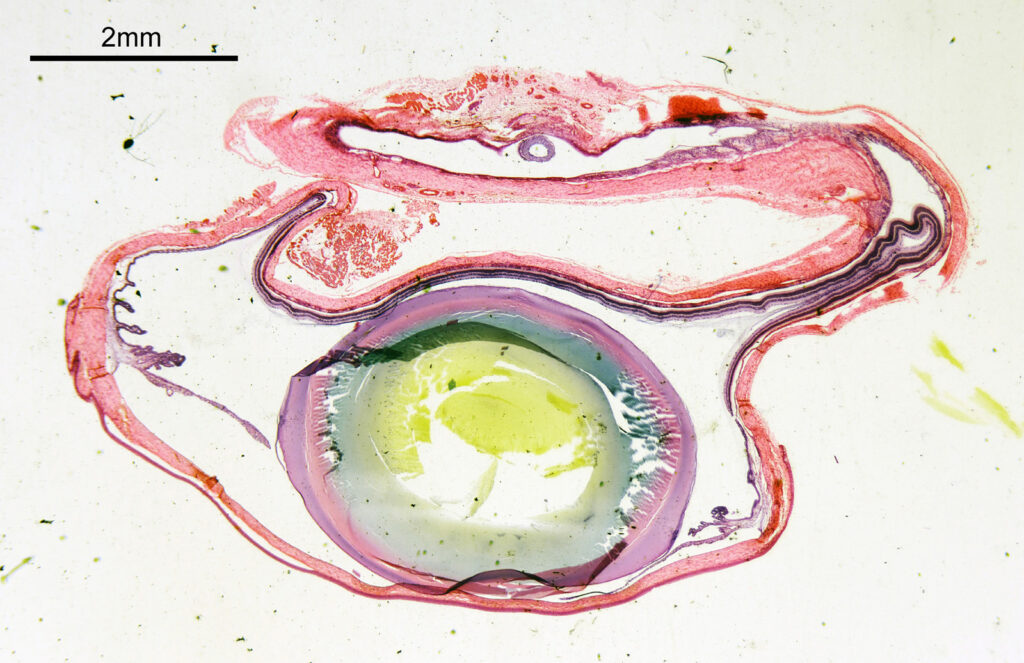
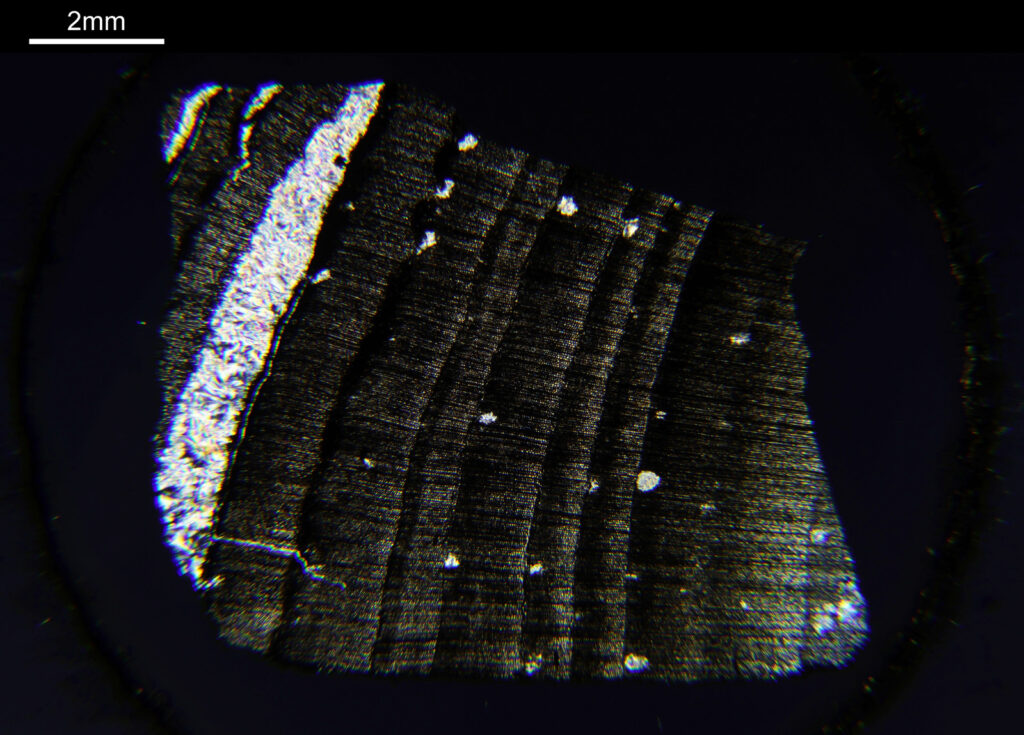
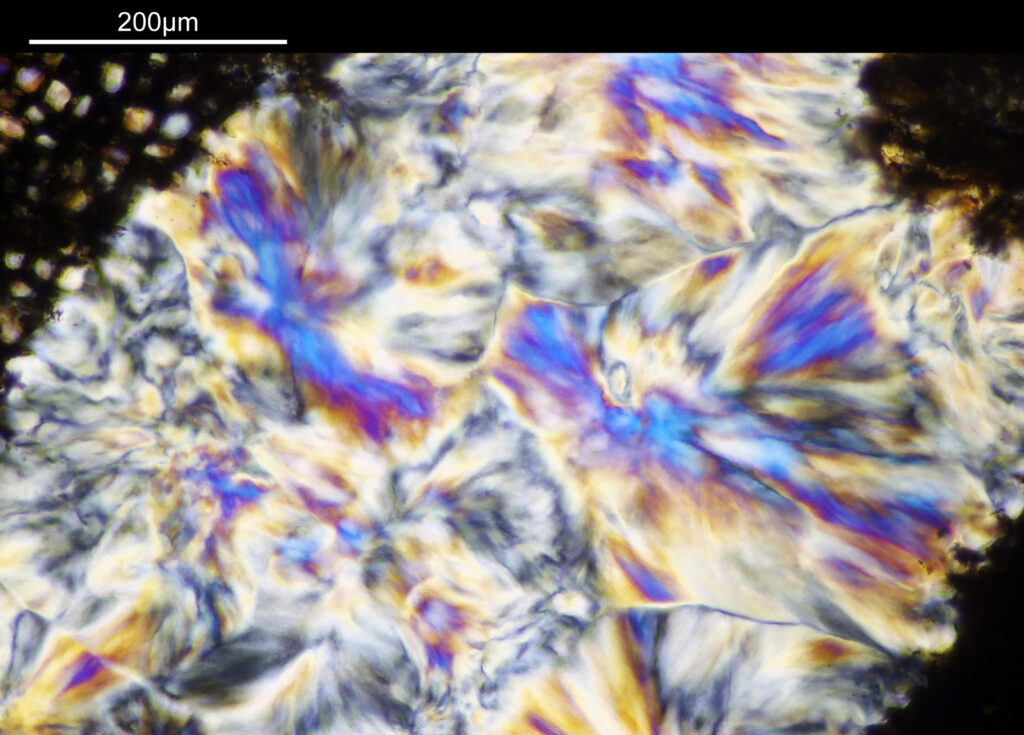
The eye itself is towards the bottom of the image (if that wasn’t obvious). The lens is the oval feature which looks purple/green/yellow. The eyeball itself is squashed here so is no longer spherical. I had hoped to look at the sample with cross polarized light to look at the construction of the lens itself, but the slide mount is crystalline and so this wasn’t possible. Note to self, keep looking for an older sample in case that doesn’t have a crystalline mount.
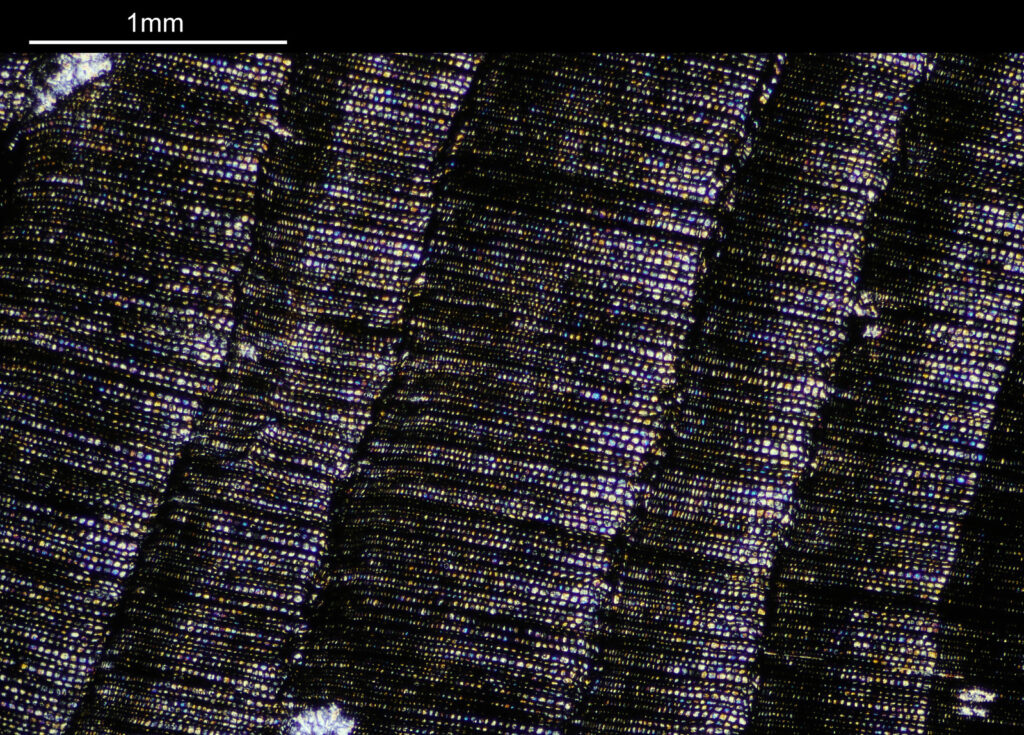
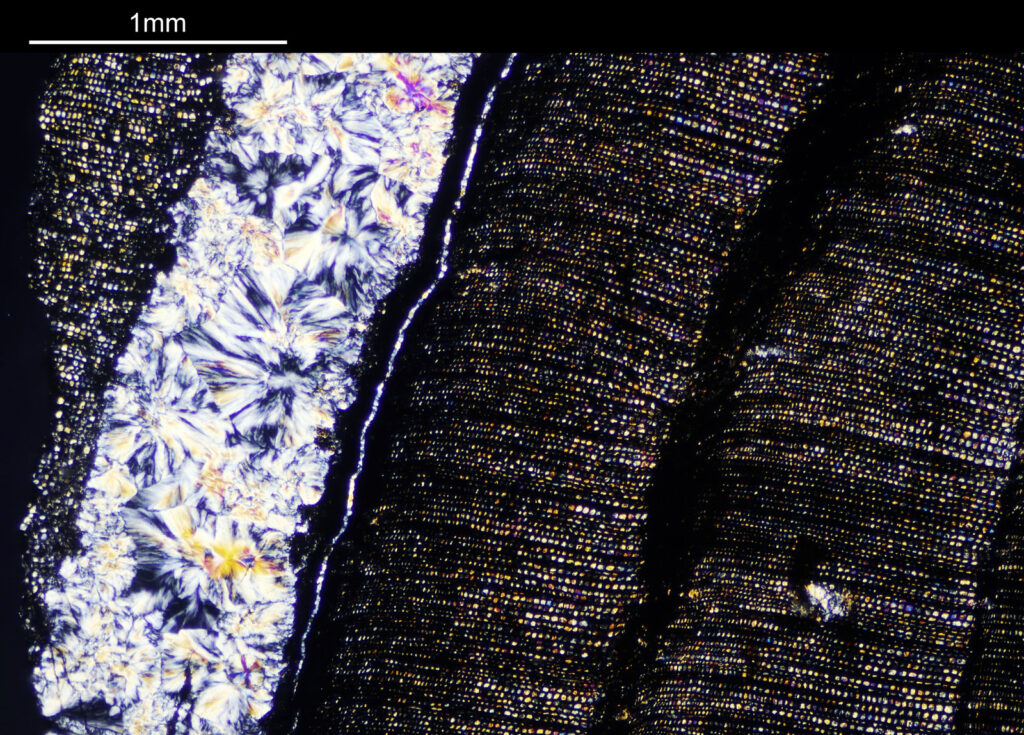
How about if I look at the sample with a 40x Olympus Dplan Apo UV objective, what can be seen? Images using this higher magnification are given below.
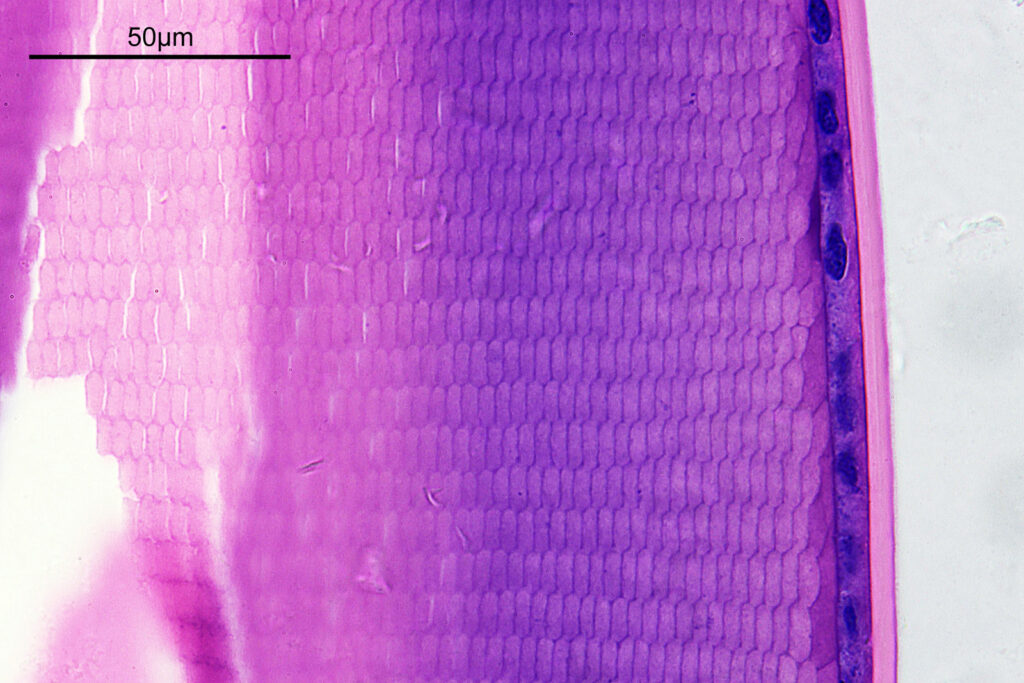
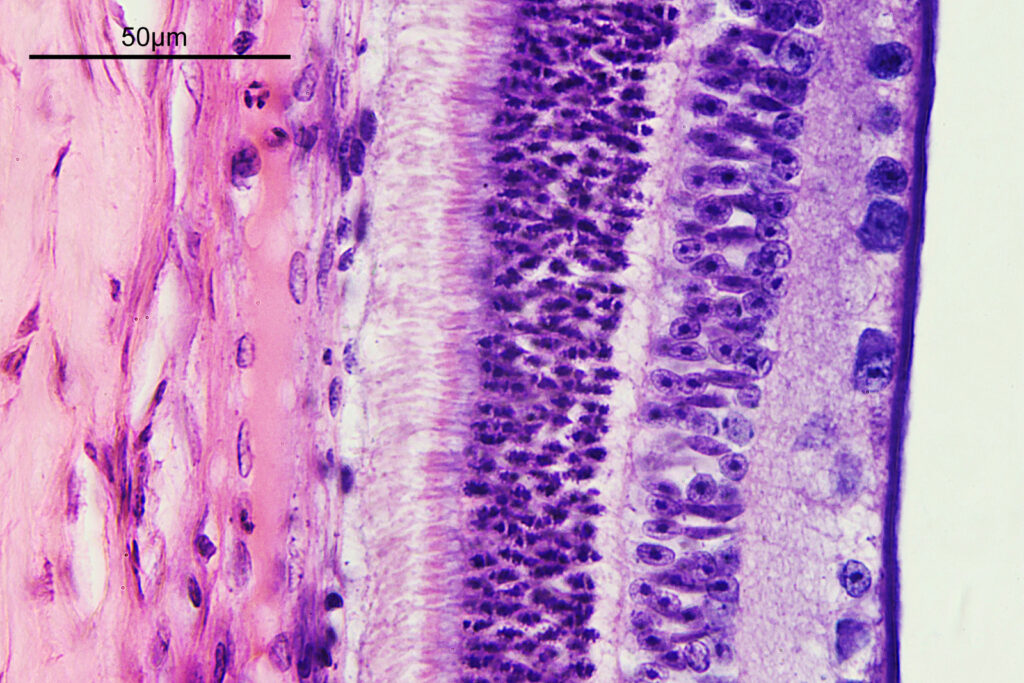
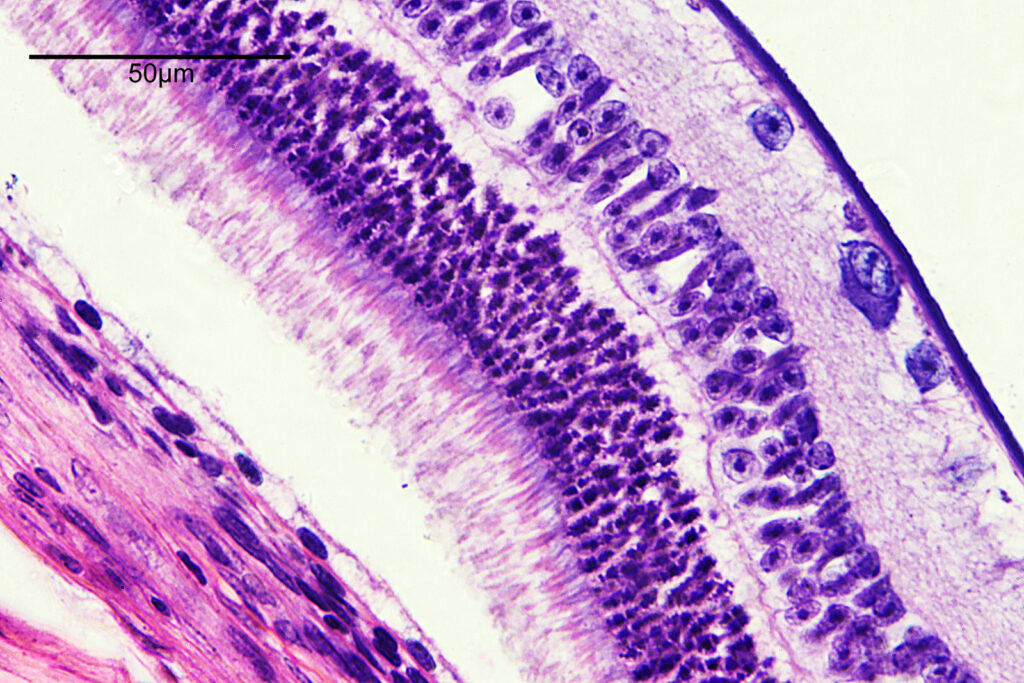
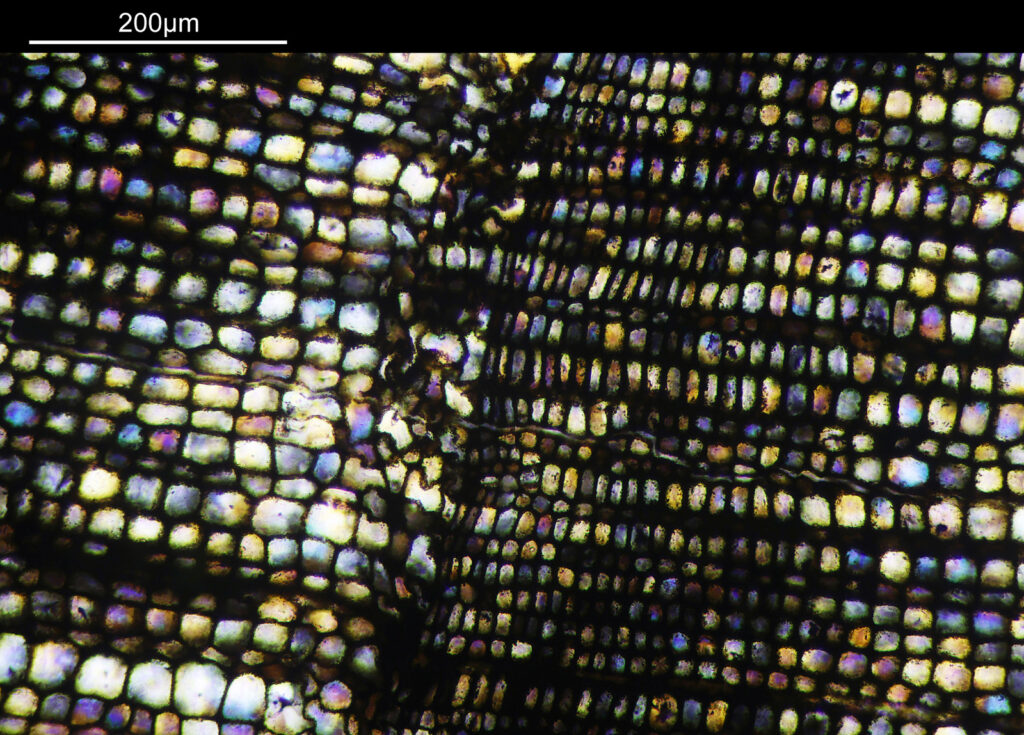
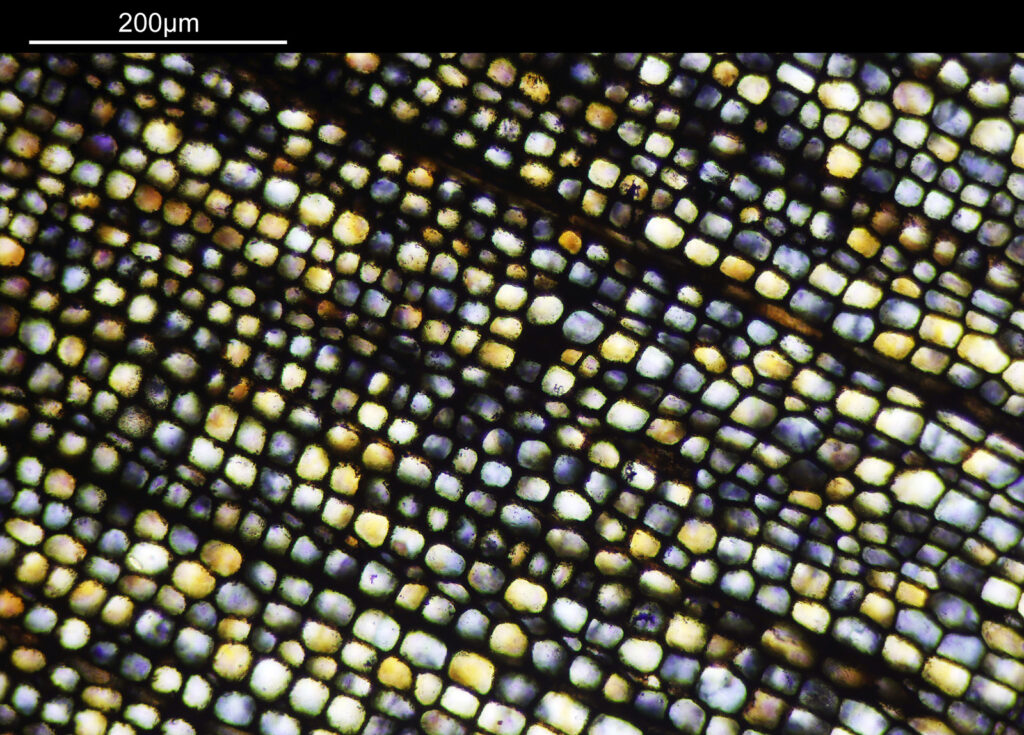
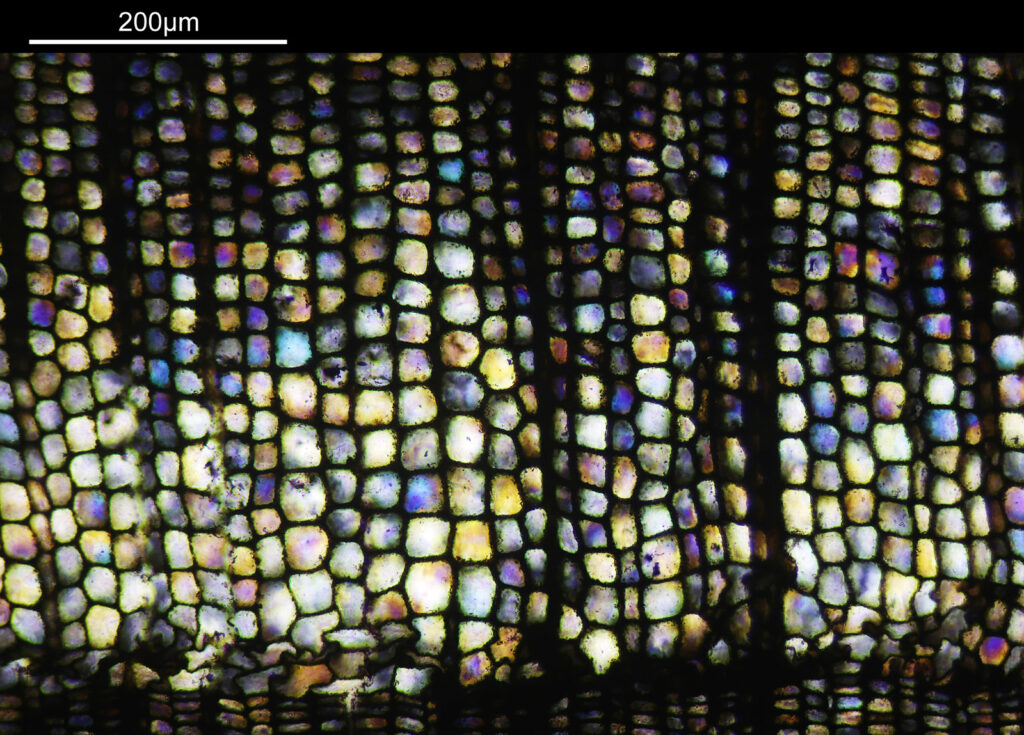
There are some beautiful features in the sample of the slide. What are we looking at though? In the first of the three images above it is showing the lens of the eye and you can see the cross section through the lens fibers. I had hoped to look at this using cross polarised light, but as mentioned this wasn’t possible due to the mount used to make it.
The second and third images are the tissue at the rear of the eye – the retina region. I found a nice paper which shows the different layers present (The Role of Photodynamic Therapy in Non-malignant and Malignant Eye Disorders, by Nowak-Sliwinska et al.). I took one of my images, flipped it horizontally and put it below their schematic.
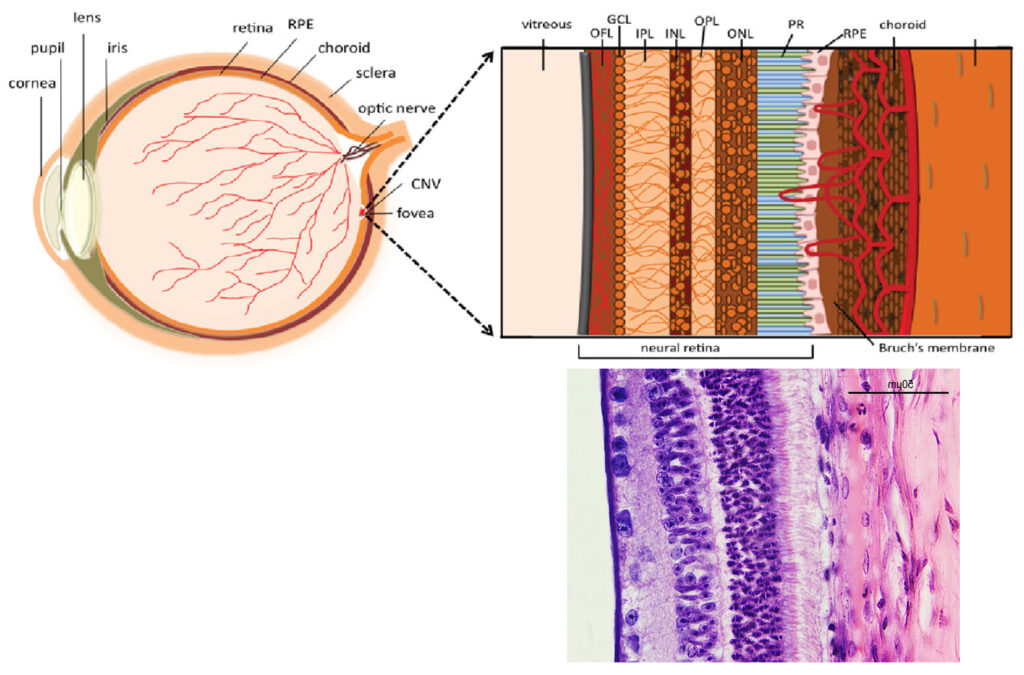
Although slightly different thicknesses, the schematic lines up quite well with my image. It’s interesting to see the rods and cones in the retina, and it makes me realise how complex it is back there.
I did return later to the slide and looked at it with my 60x Olympus Splan Apo NA 1.4 objective, and my monochrome Nikon d800. This was done with white LED light and is a composite of 2 images stitched together. It has been hugely reduced in resolution for sharing here – this one is 1600×517 pixels, while the original was 14540×4701 pixels.
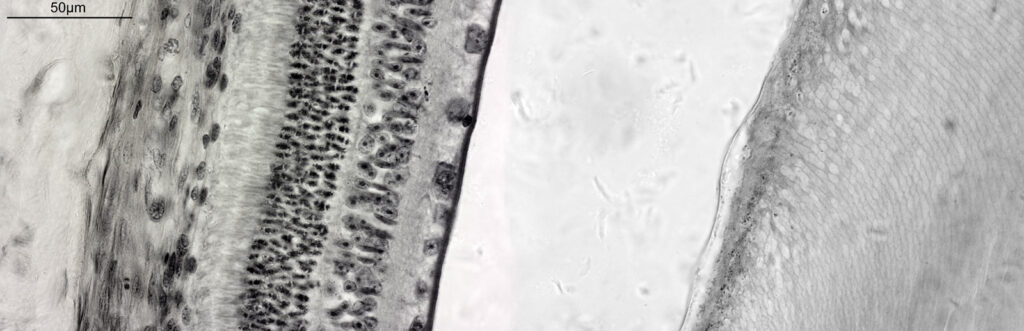
The image above shows the tissue from the rear of the eye (the retina) on the left, the remains of the vitreous humor in the middle of the image, and actually part of the eyeball lens itself on the right (the features you can see in there are the lens fibers). I had hoped to get more detail on the rods and cones, but wasn’t able to do that here.
Overall it seems to be a nice little slide, and one I will try and come back to with higher magnification to look more at the rods and cones. As always, thanks for reading, and if you’d like to know more about my work I can be reached here.