Today’s post contains the images from four diatom slides by the slide maker William Allott Firth (W.A. Firth) who is widely renowned as one of the finest slide makers there has been. For more information about W.A. Firth, take a look at the excellent writeup here. These were taken using my modified Olympus BHB microscope and 450nm LED light and have been stacked using Zerene. All have been reduced in resolution for sharing as the original file sizes are too big to upload efficiently.
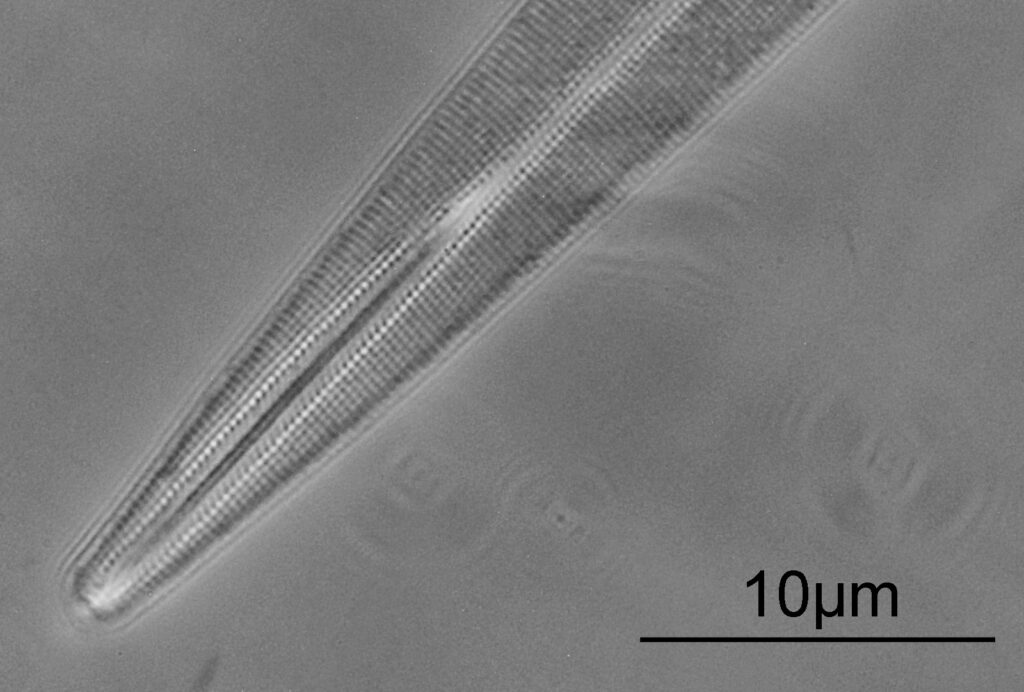
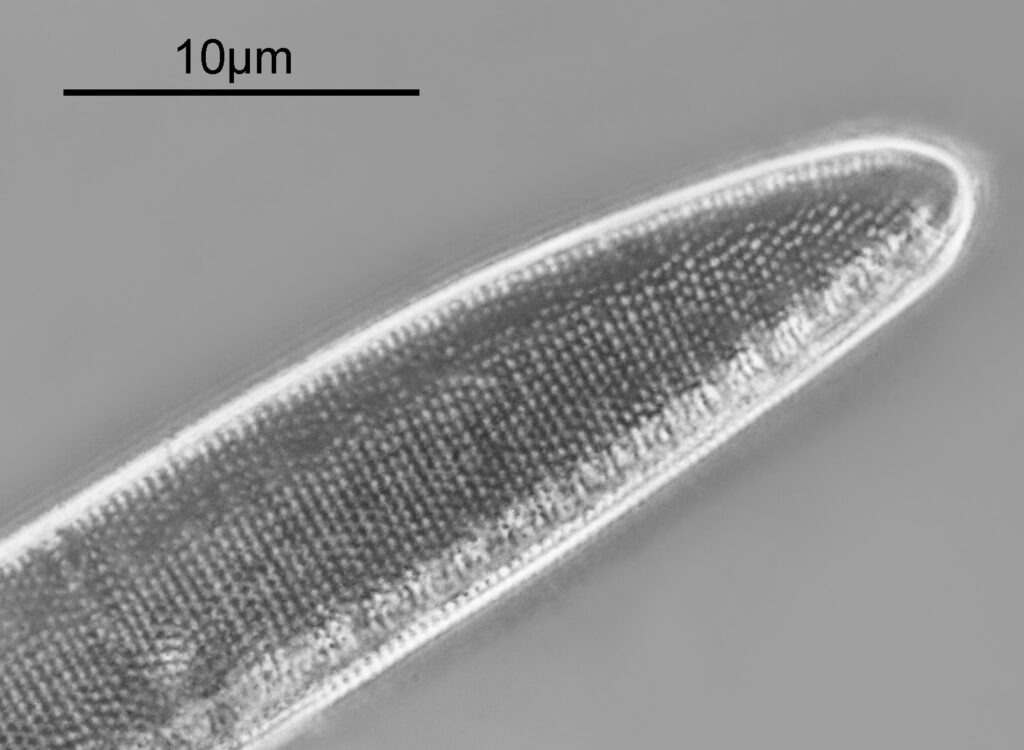
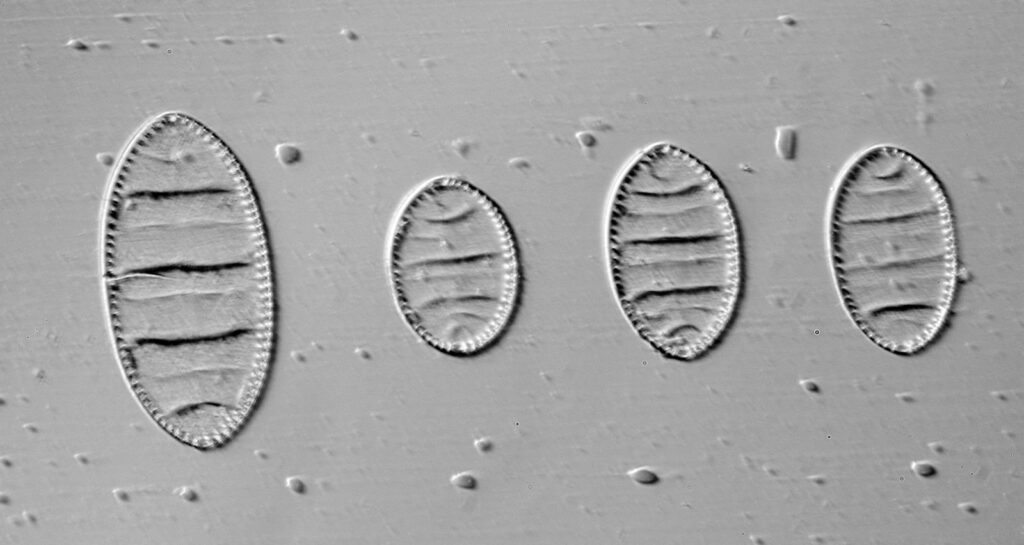
Opephora schwartzii
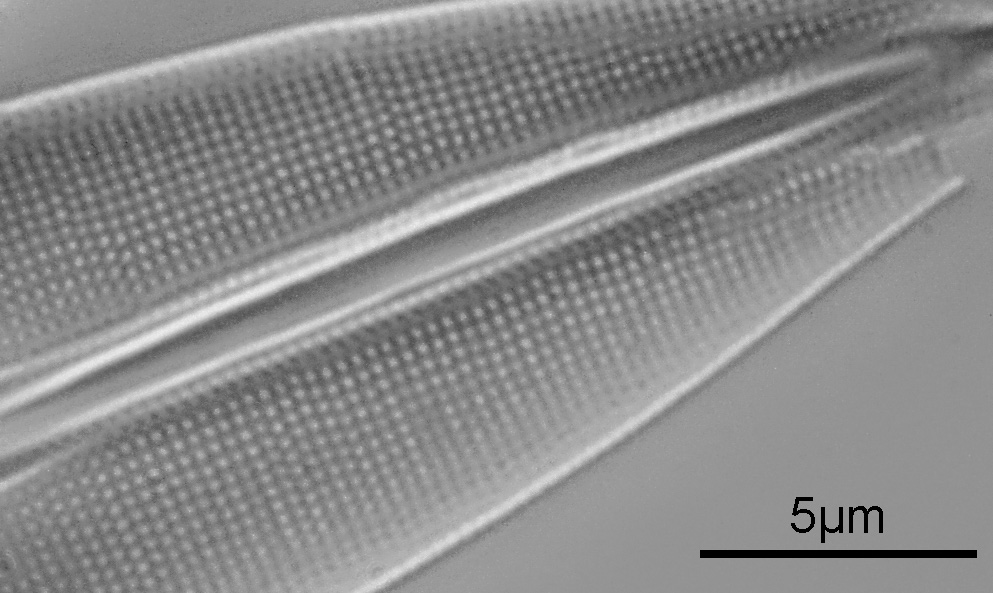
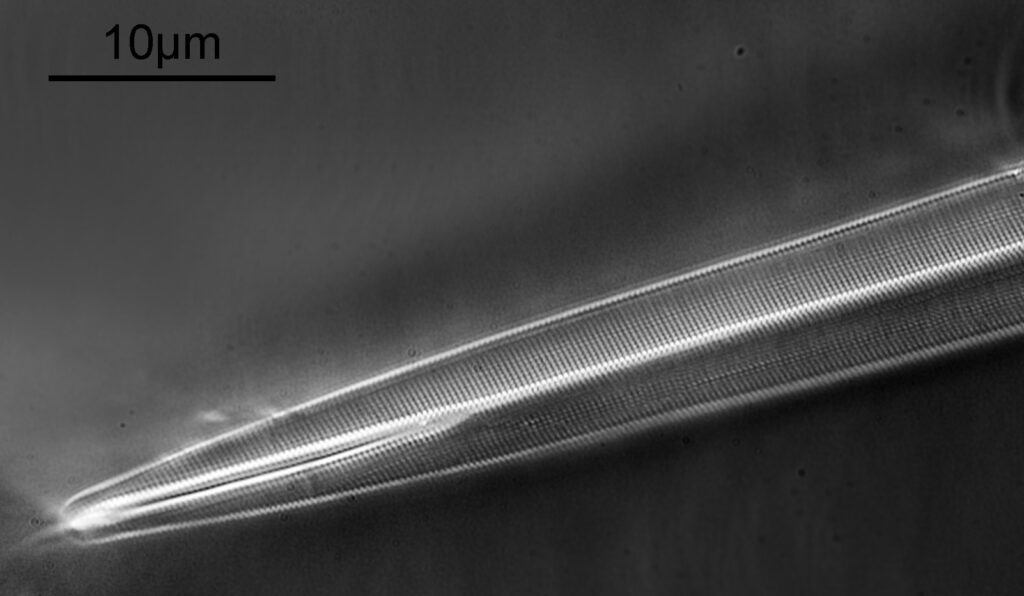
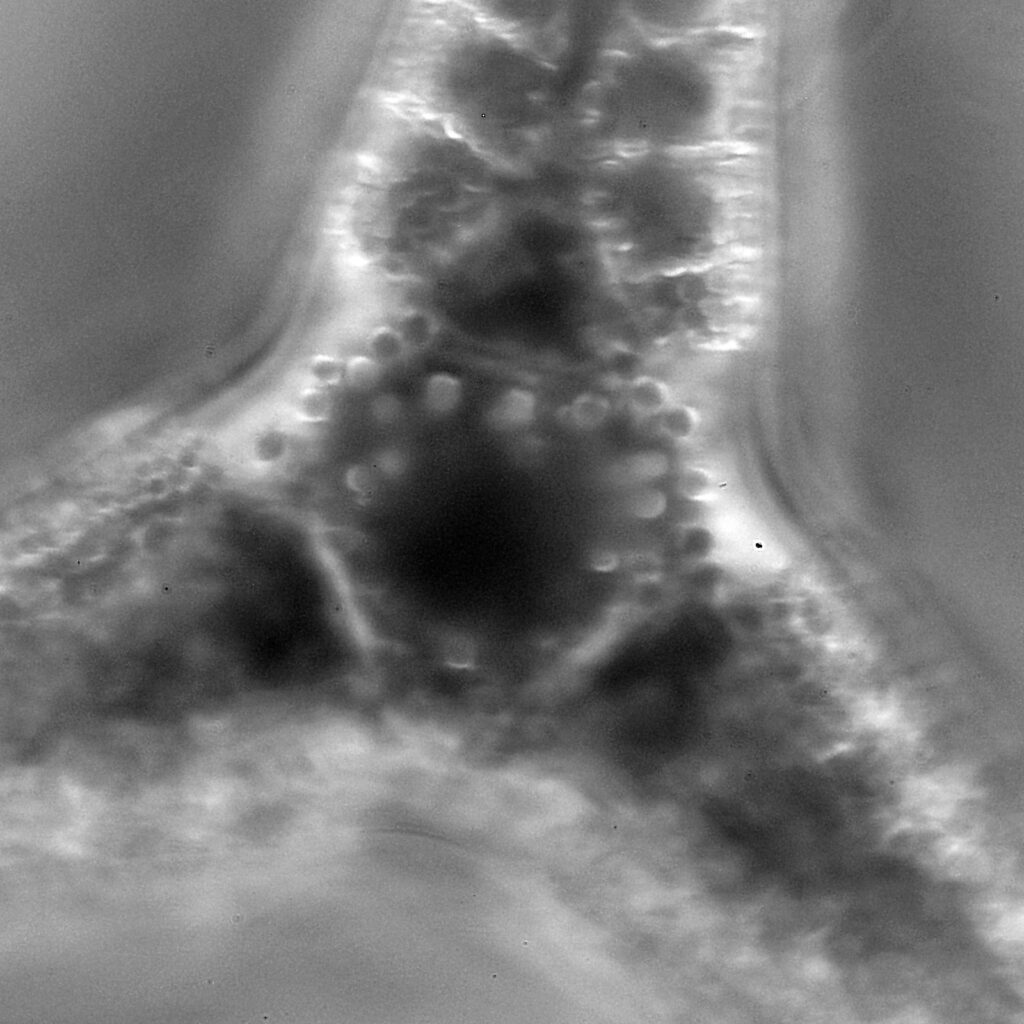
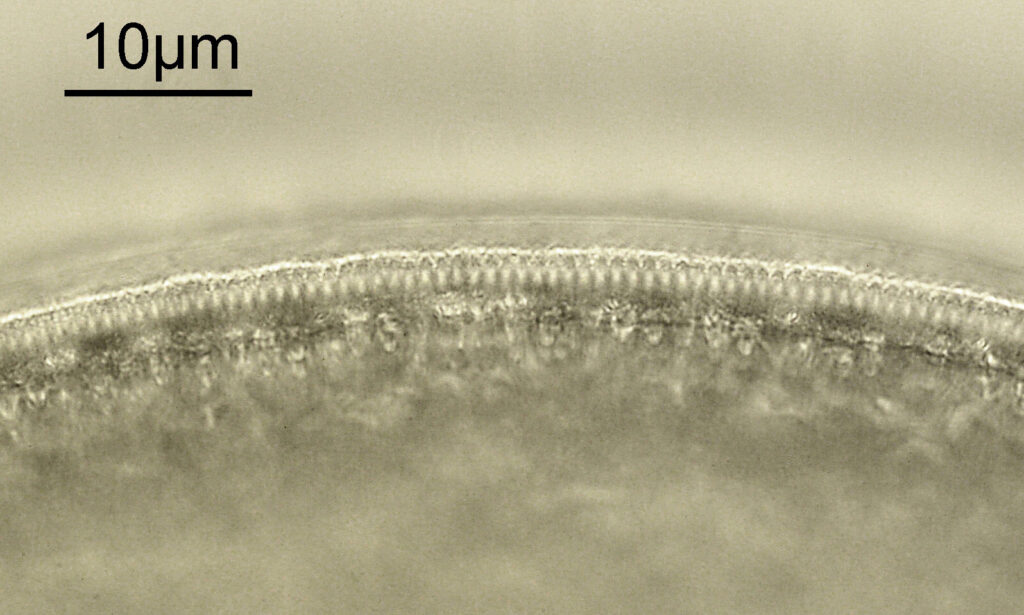
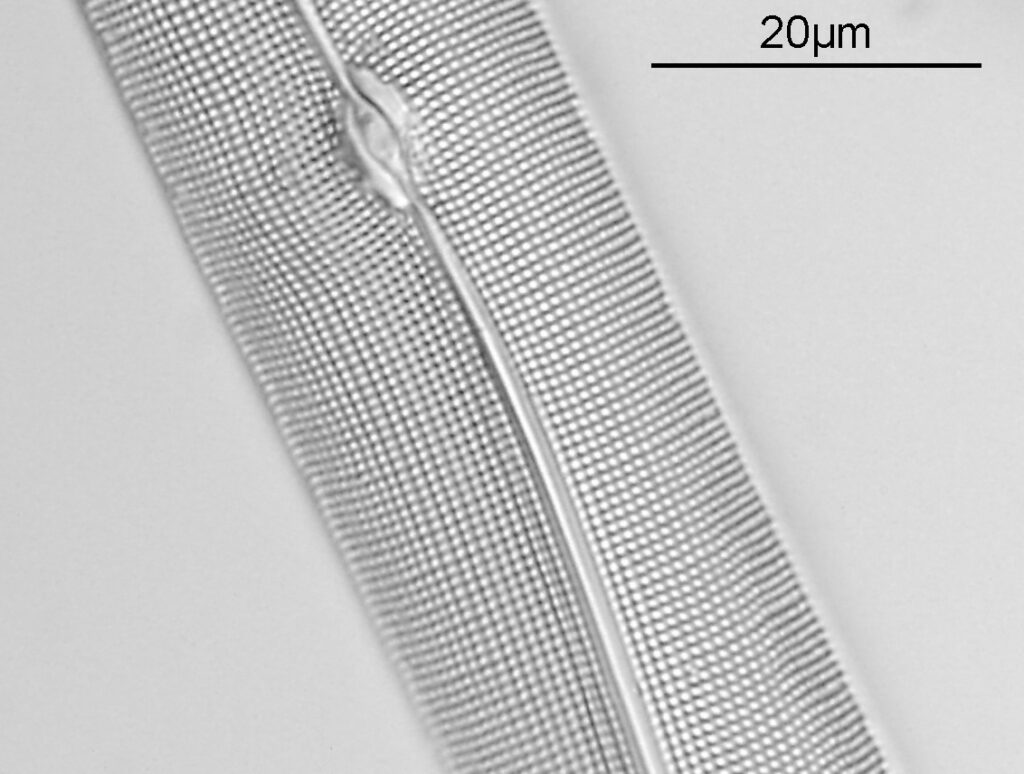
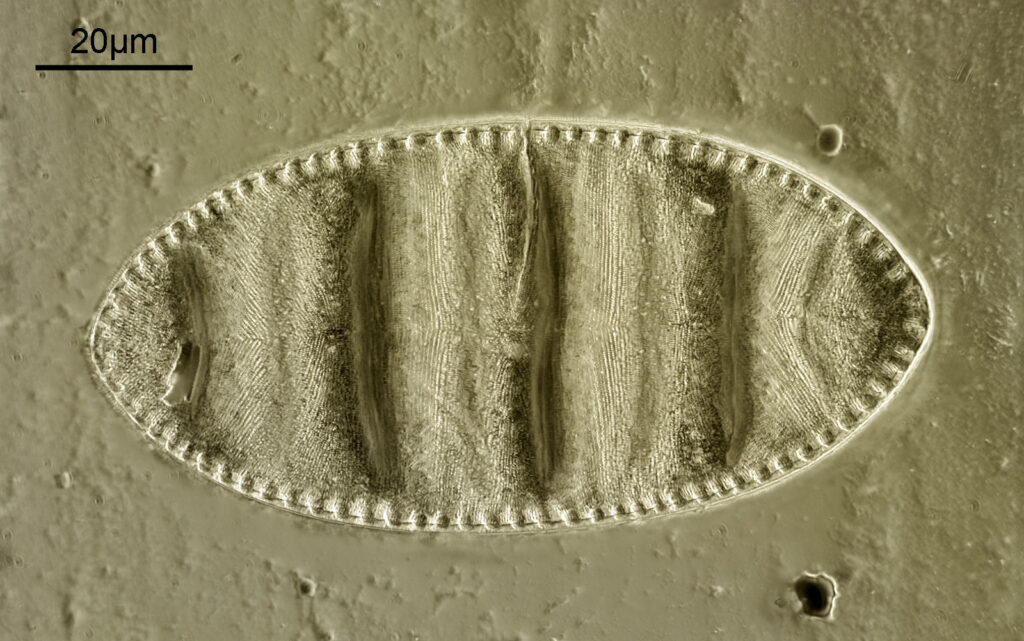
The first one is Opephora schwartzii. This was imaged using dark ground, using a 100x Leitz Pl Apo NA 1.32-0.60 objective with oil immersion, and a Reichert Neo 1.42/1.18 dark ground condenser. The iris on the objective was closed to make the dark ground image. There are 2 examples of the diatom on the slide.
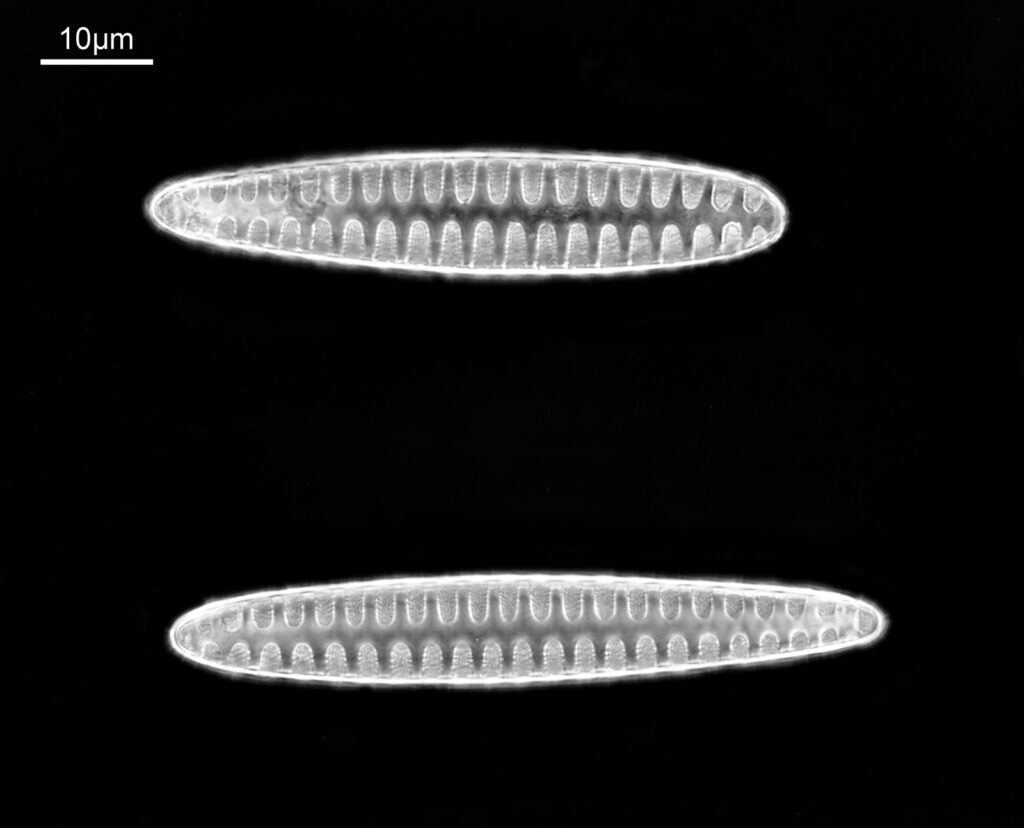
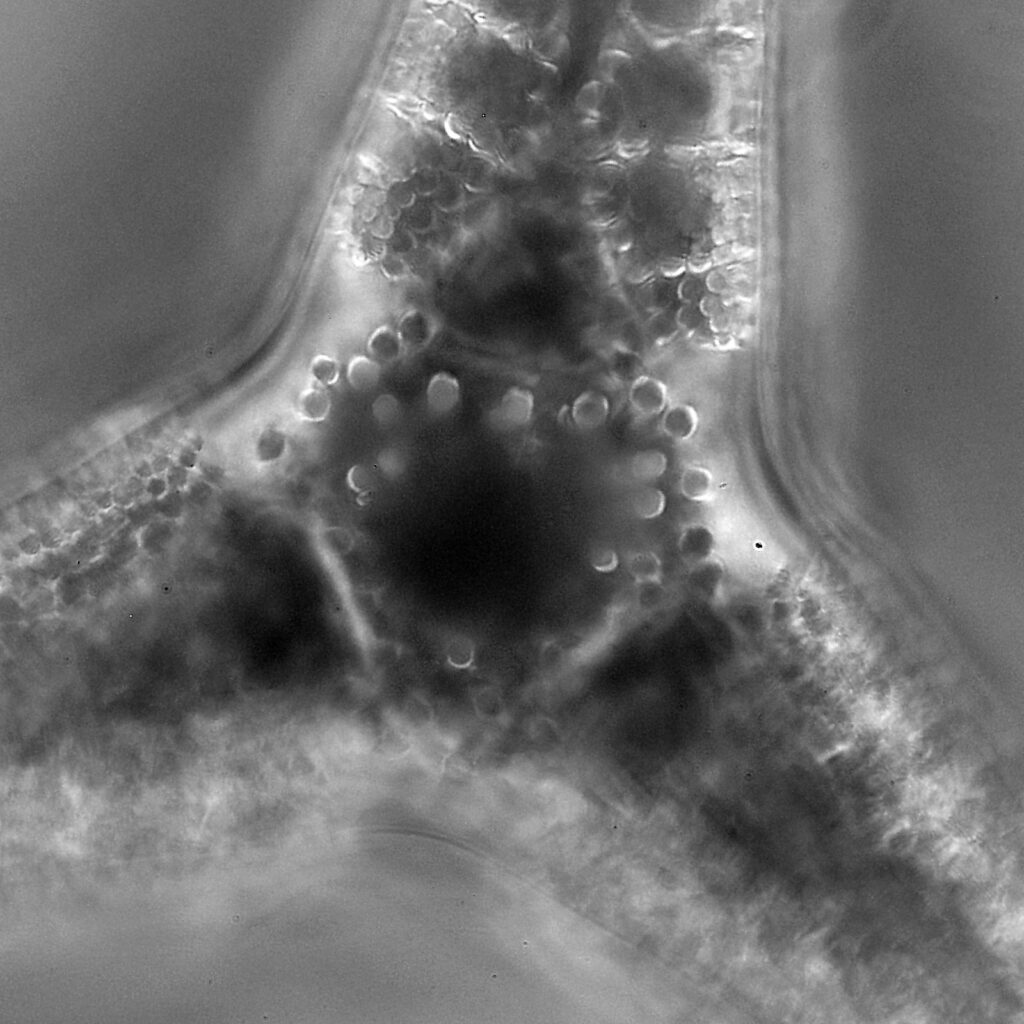
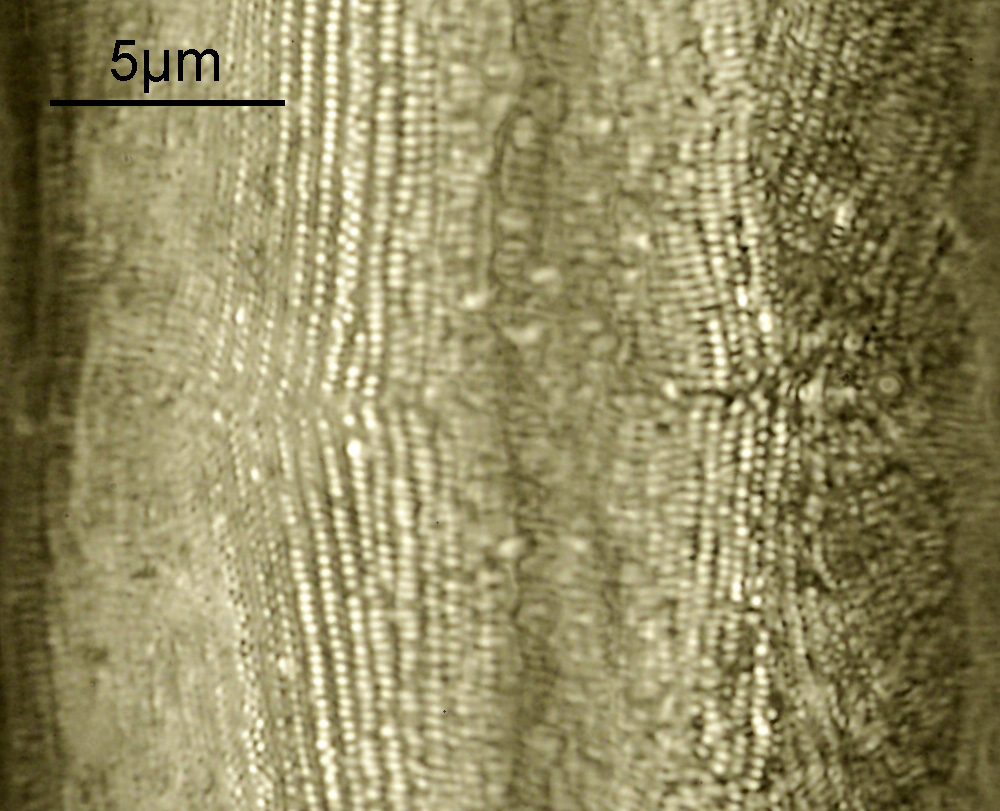
These were small diatoms, but even so the original has been reduced in size for sharing, and some of the details are lost in the image above. Below is a crop of the image shown at the original pixel resolution.
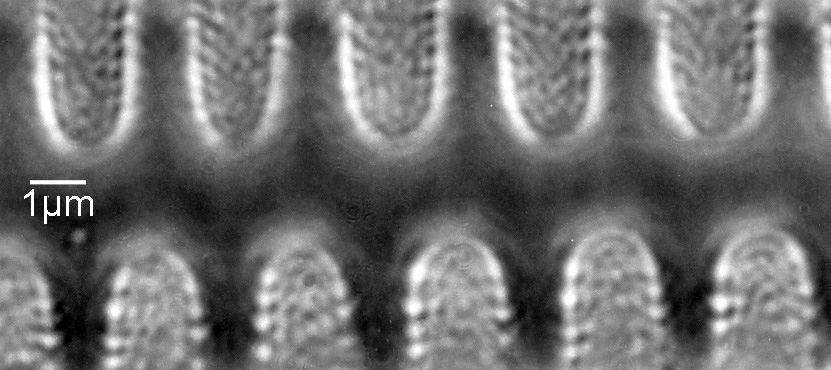
The dark ground image has done a good job of bringing out some of the sub-micron structures in the diatom. Got to love pushing the limits of optical microscopy. Before I move to the next one here’s the slide.

Aulacodiscus orientalis
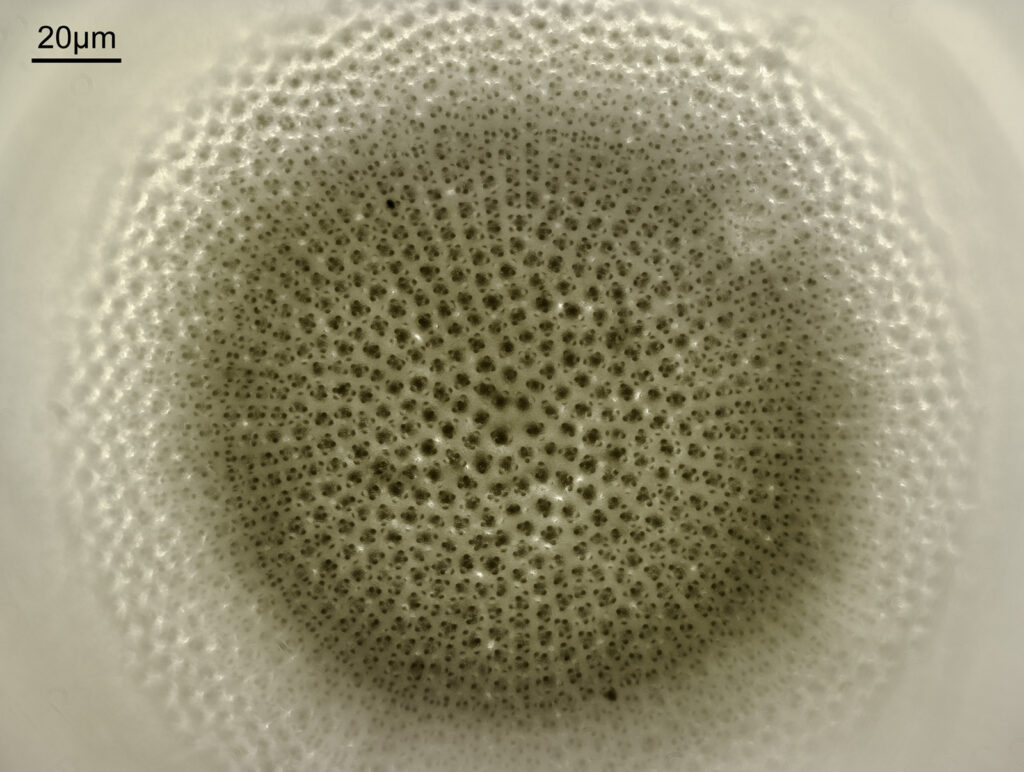
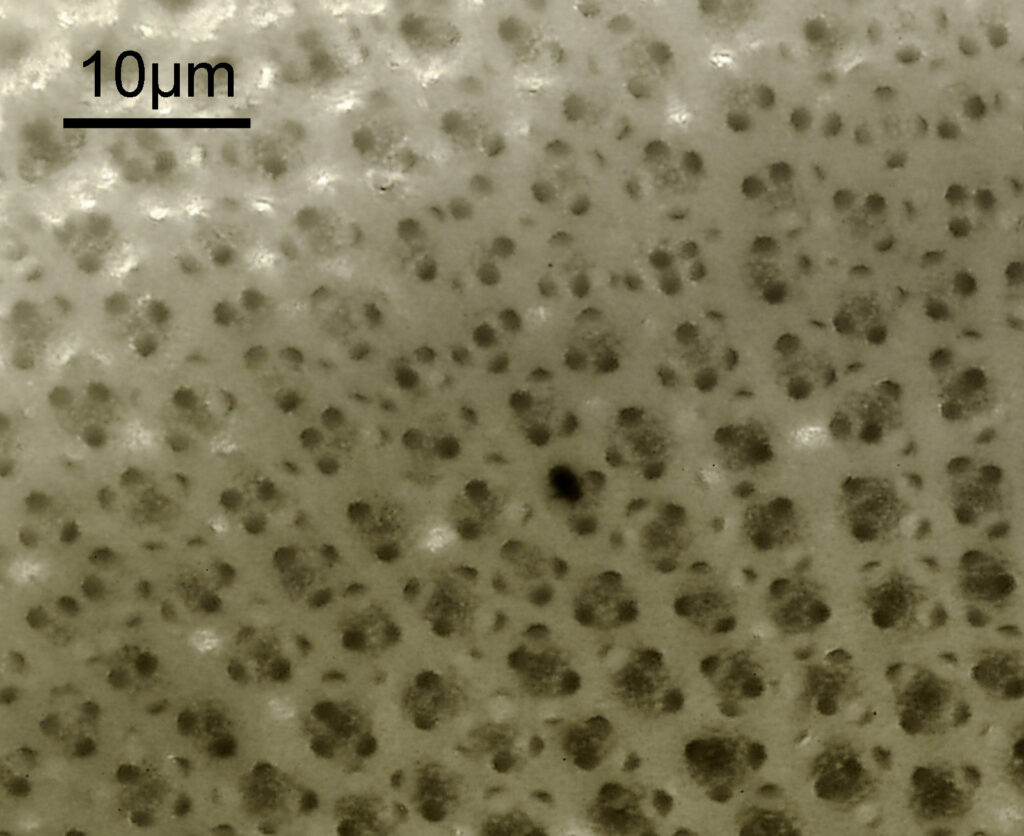
Next is Aulacodiscus orientalis. This was imaged using brightfield with an Olympus Aplanat Achromat condenser (oil immersion), using a 40x Leitz Pl Apo NA 1.00 objective with oil immersion.

Here’s the slide.

This one confused me a bit at the beginning as it looked like it said ‘Hulacodiscus’ for the name.
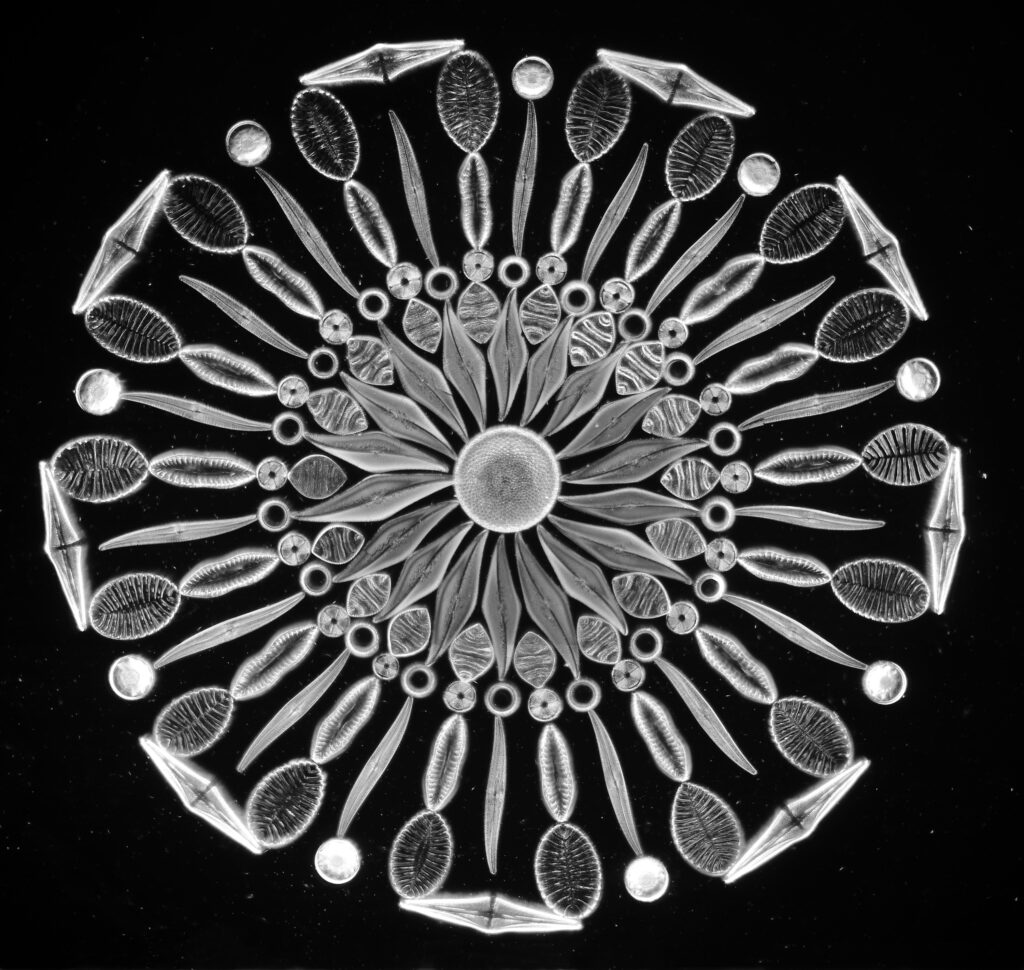
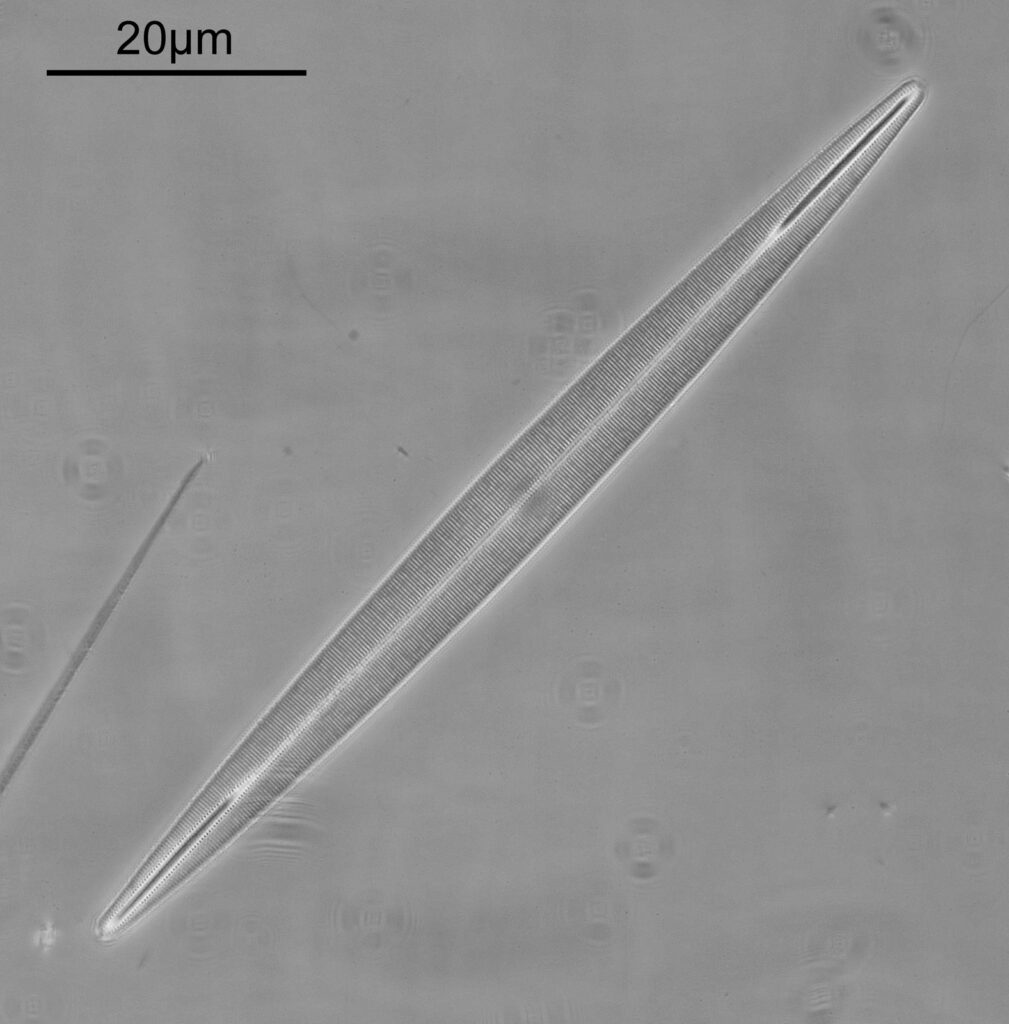
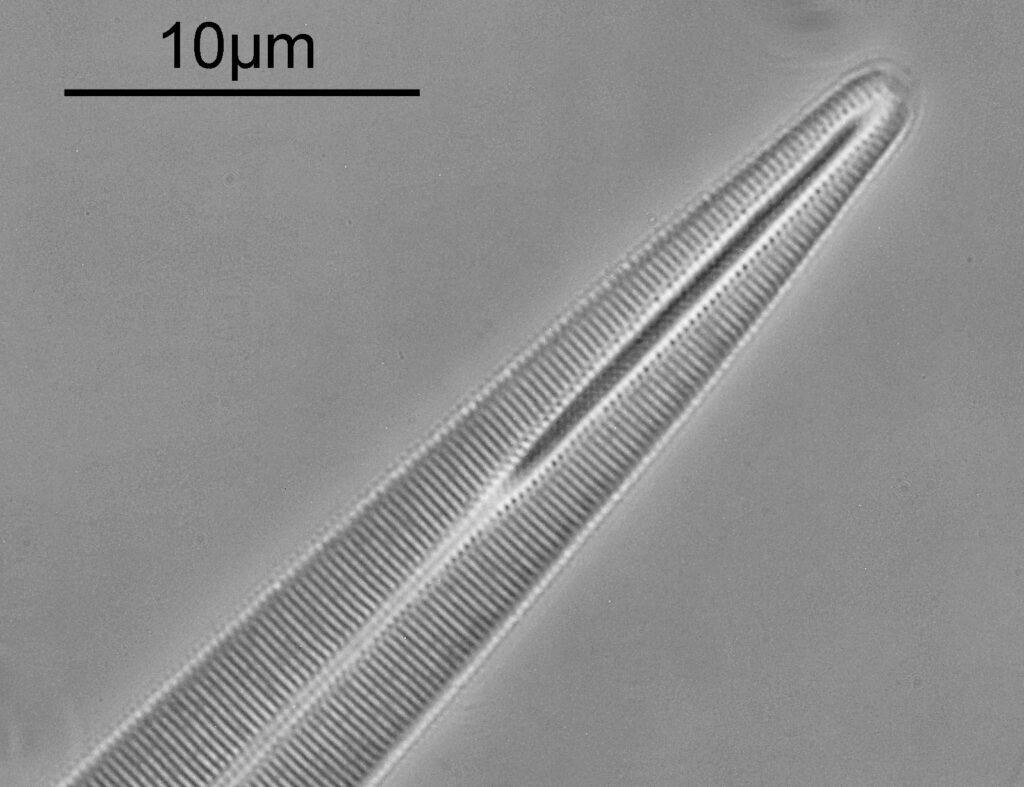
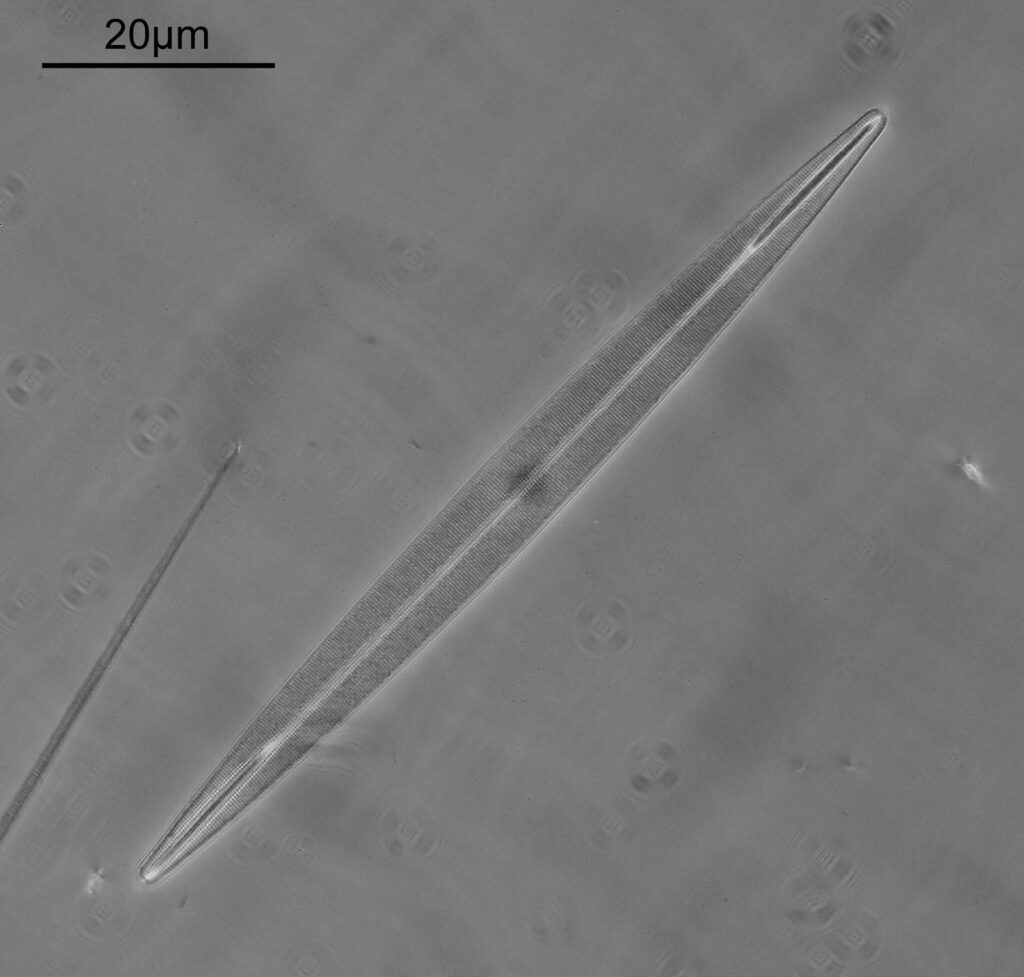
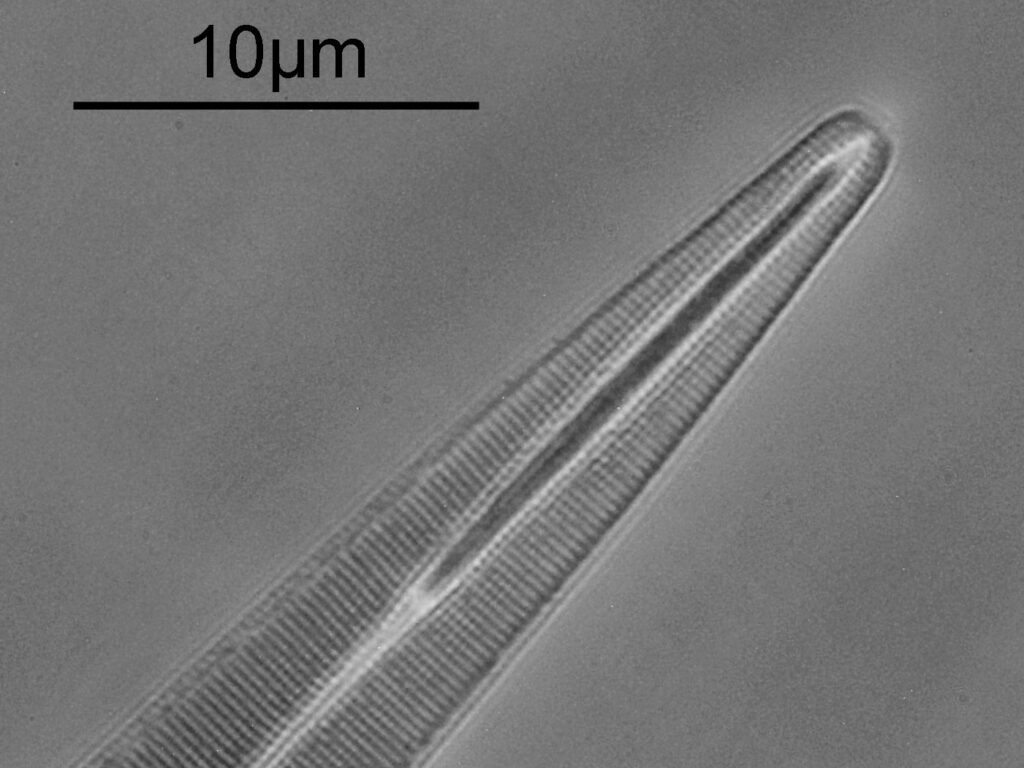
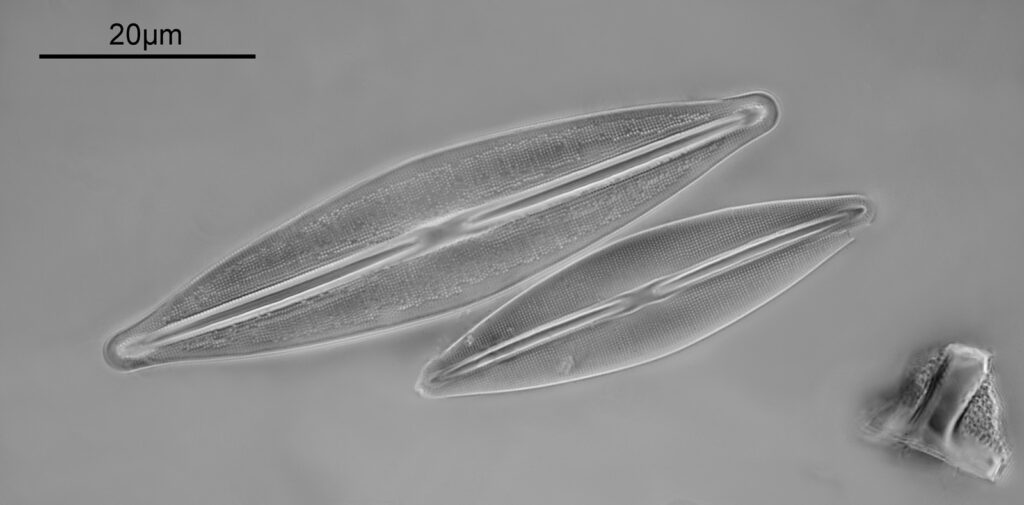
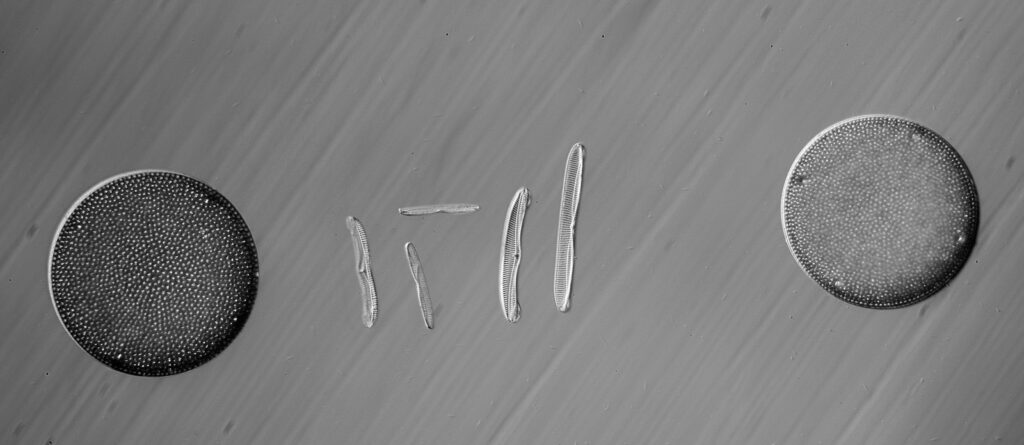
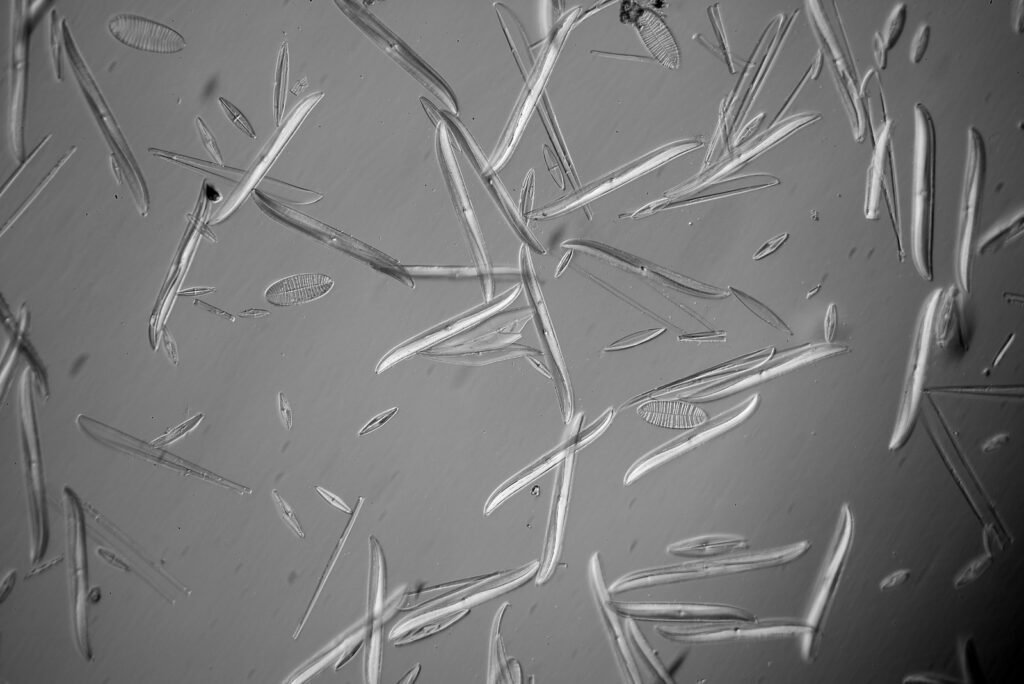
Surirella macraeana
The next slide is Surirella macraeana. Back to a smaller diatom, and a 100x Leitz Pl Apo NA 1.32-0.60 objective was used (with oil immersion), and the iris closed down slightly to make circular oblique lighting. A Leitz Heine condenser was used along with oil immersion.
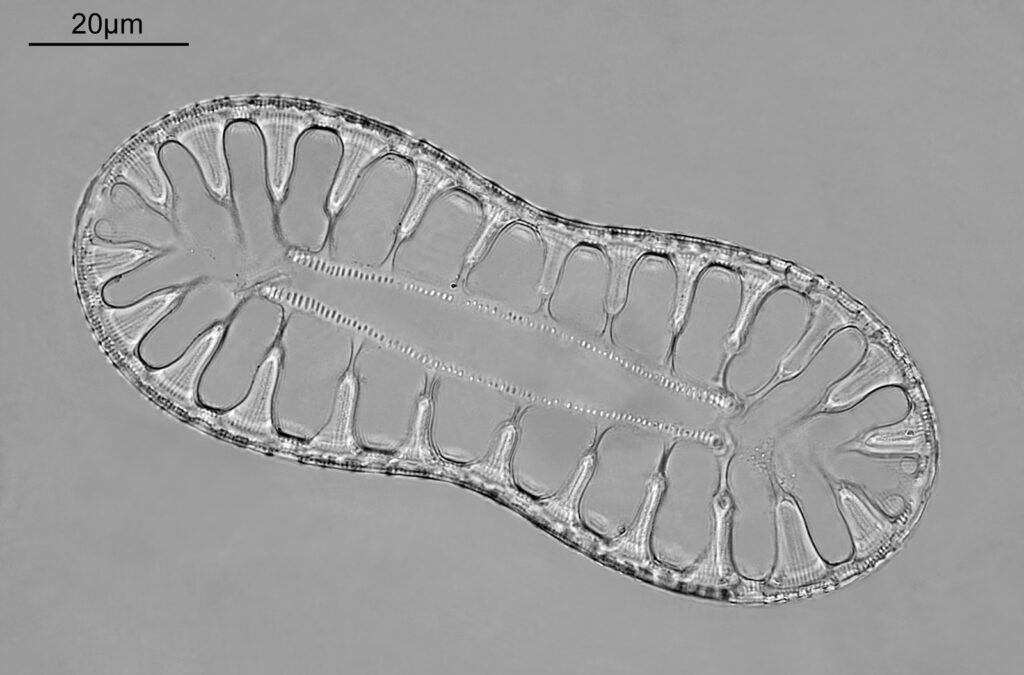
There were 6 examples of this diatom on the slide.
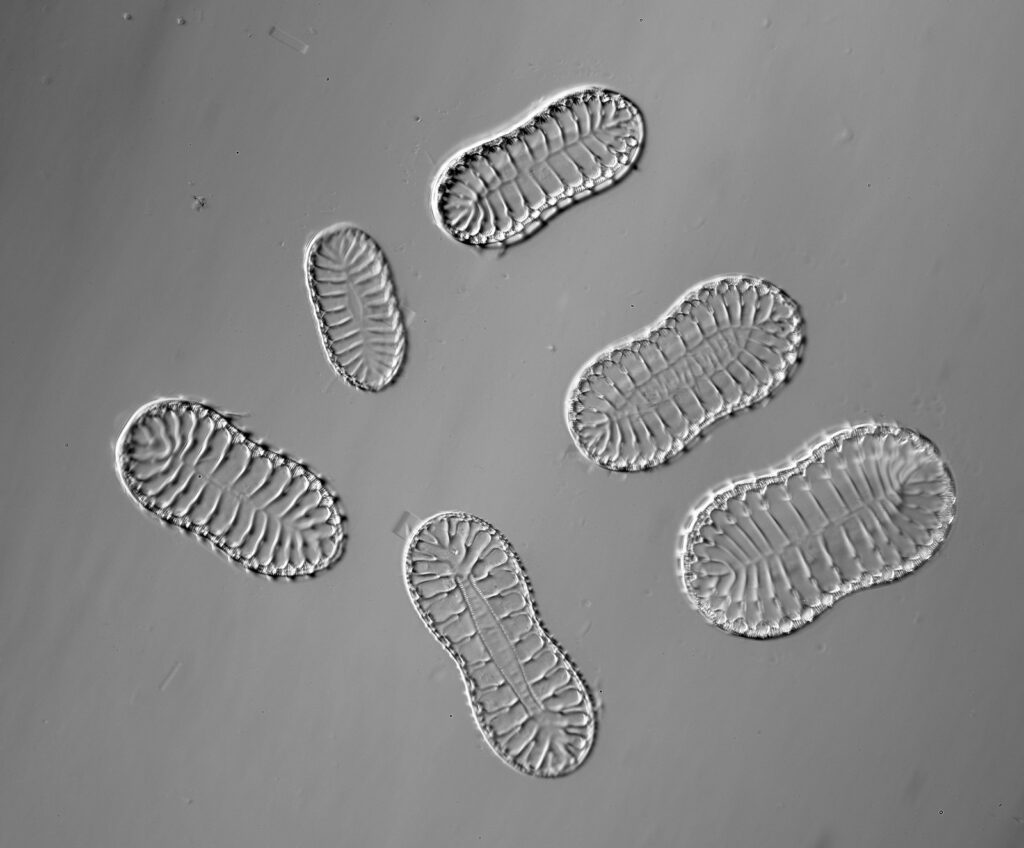
And of course the slide.

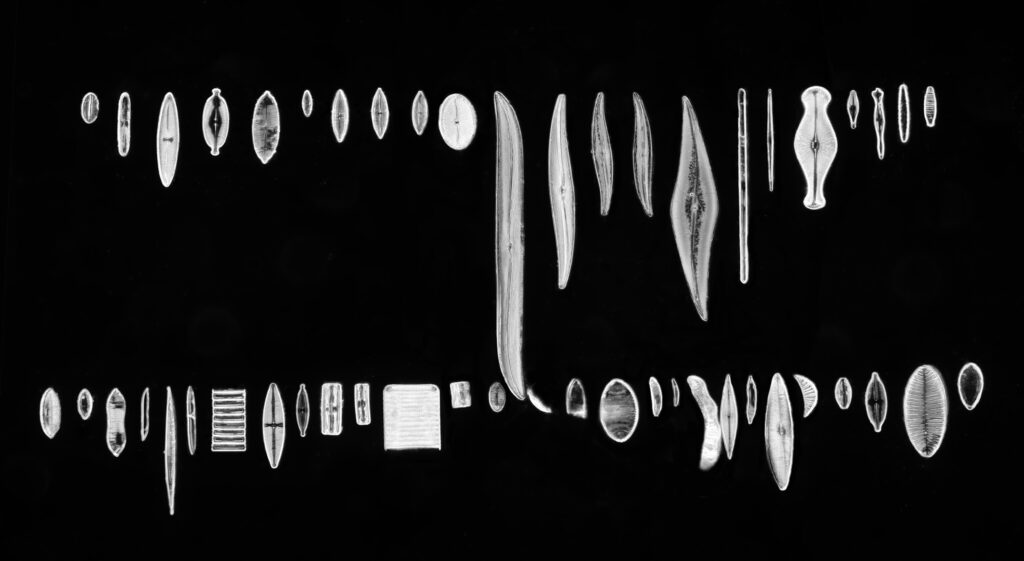
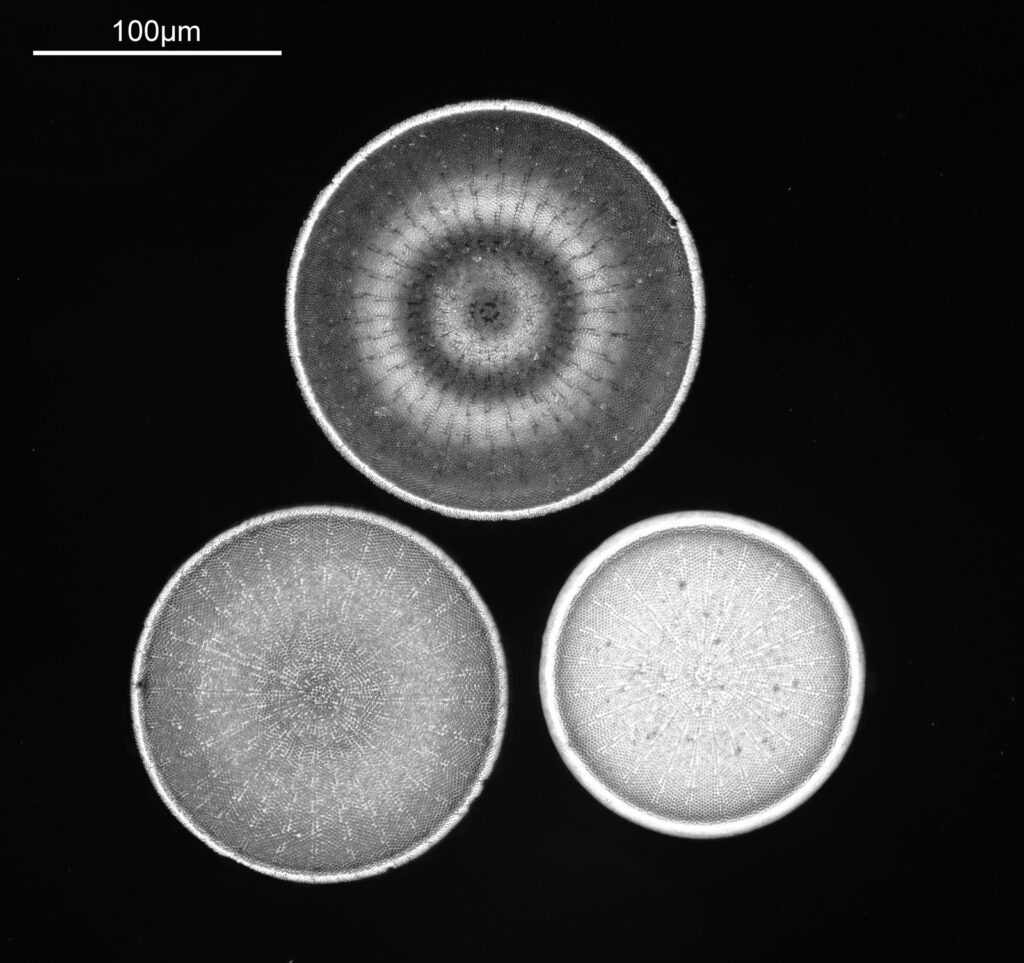
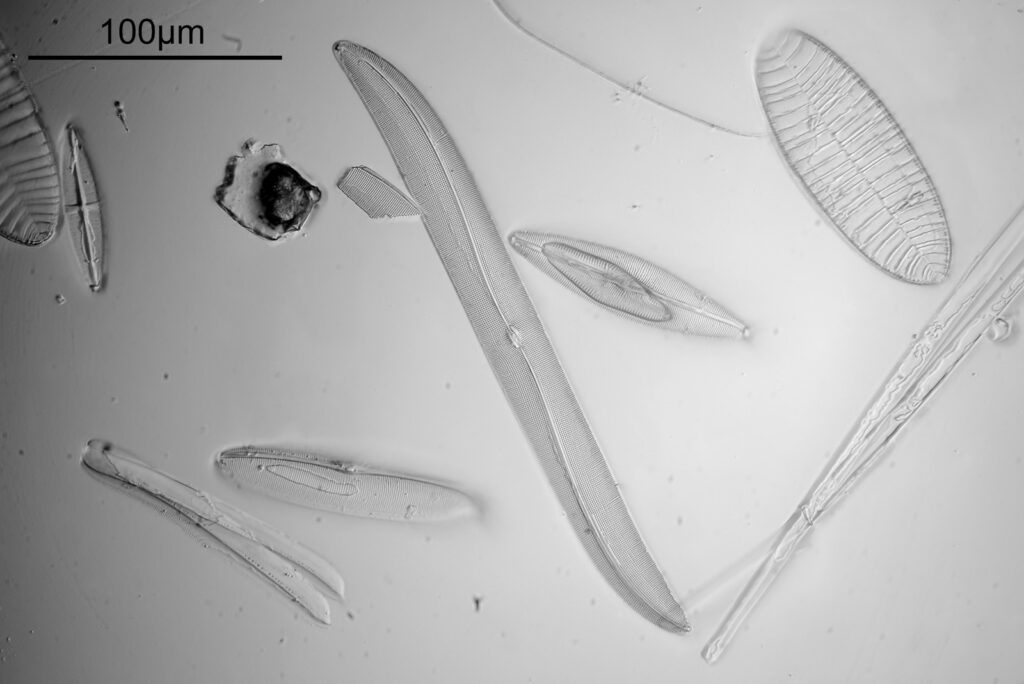
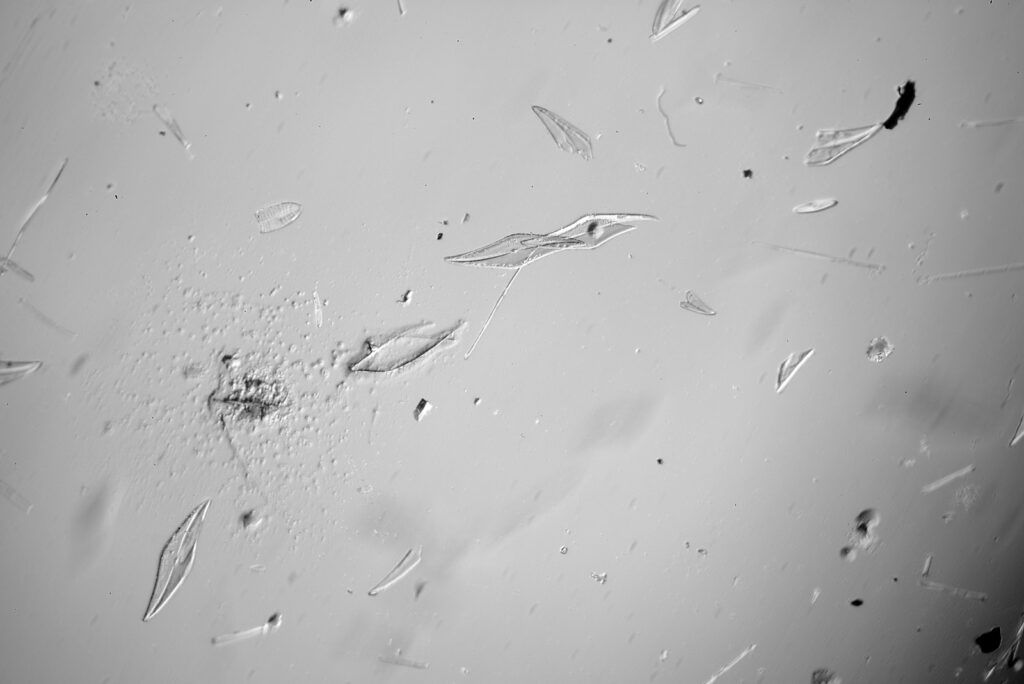
Stephanopyxis corona
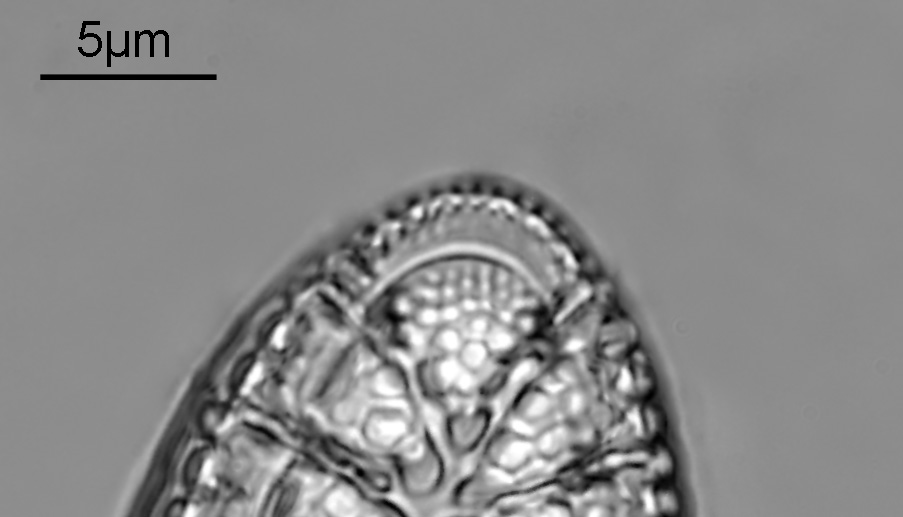
The final one today is Stephanopyxis corona. This was imaged with a 40x Leitz Pl Apo NA 1.00 objective, oil immersion, and an Olympus Aplanat Achromat condenser, slightly oblique, again with oil immersion.
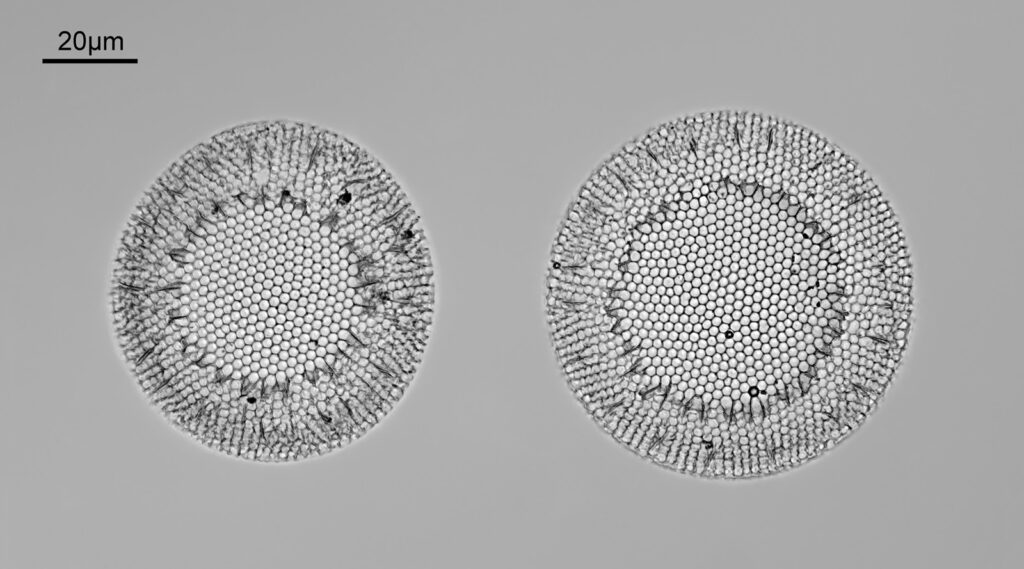
There was actually 3 examples of the diatom on the slide, but one of them had gone walkabout almost to the inner edge of the ring, as can be seen with this lower magnification image.

I had a bit of trouble selecting an objective to images this slide. They were quite small, and my original choice of 100x and 63x Leitz ones did not have sufficient working distance to reach the diatoms, and a 40x Nikon UV-F NA 1.30 couldn’t didn’t get the full depth of the diatom. Hence I ended up using the 40x Leitz, which had a slightly longer working distance. The diatoms must be quite thick (dome shaped) and this can be more easily seen if you look at a video of me moving the microscope stage.
It also proved difficult to capture the two rings of spikes in the structure in the flat photo, and they can be more easily seen in the video.
Here’s the slide.

I really enjoyed looking at these slides as their quality was so good. Amazingly, they were relatively cheap for such good quality slides – I paid about £15 for them. As always, I hope you enjoyed the images, and thanks for reading. If you’d like to know more about my work I can be reached here.