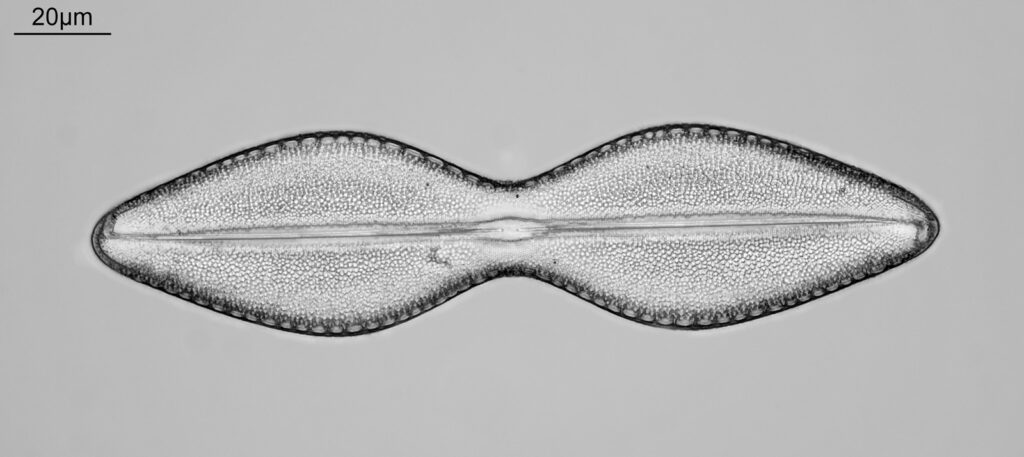
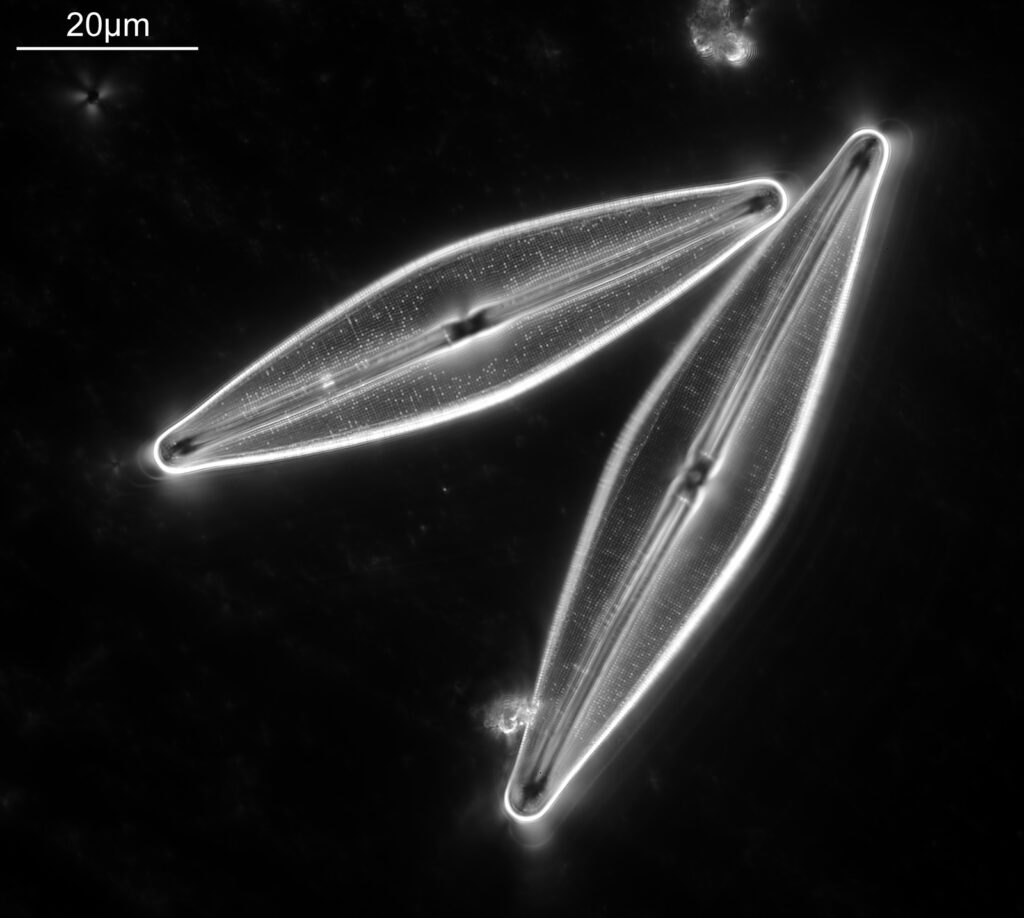
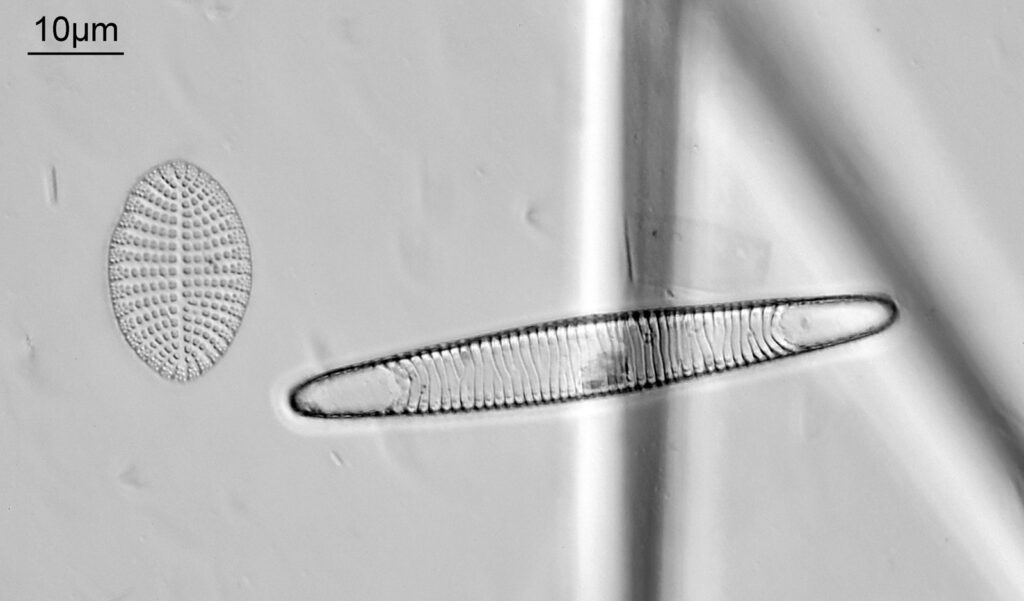
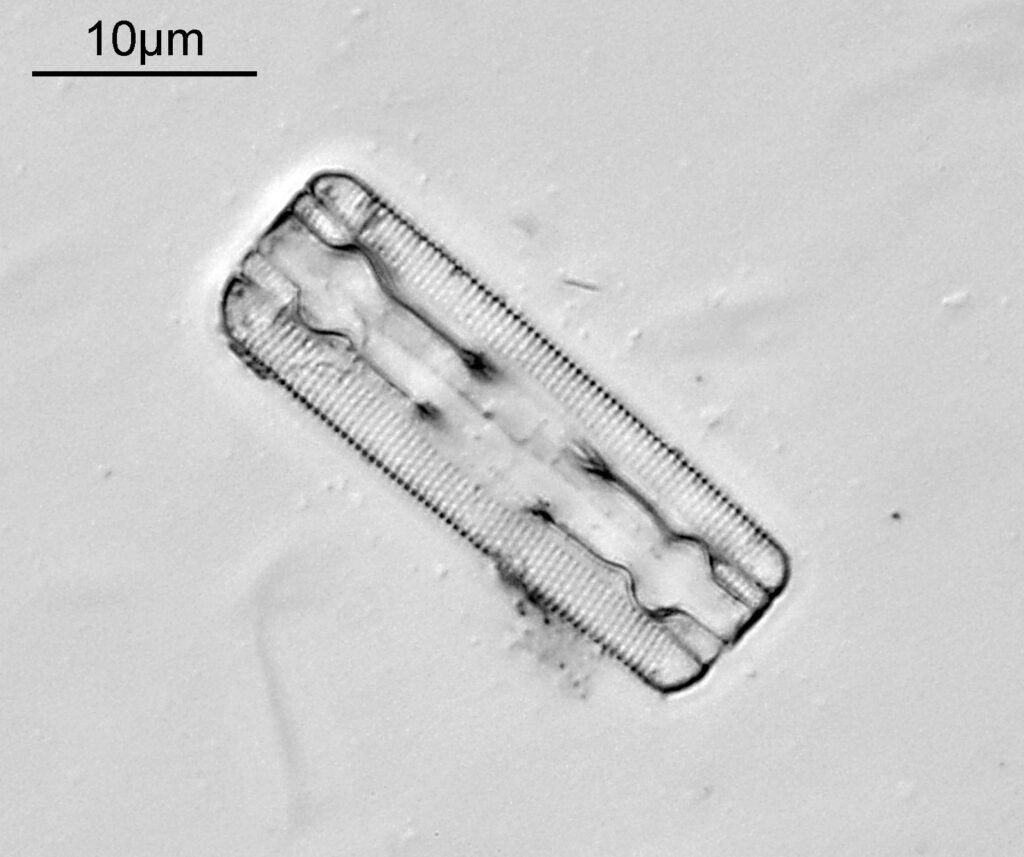
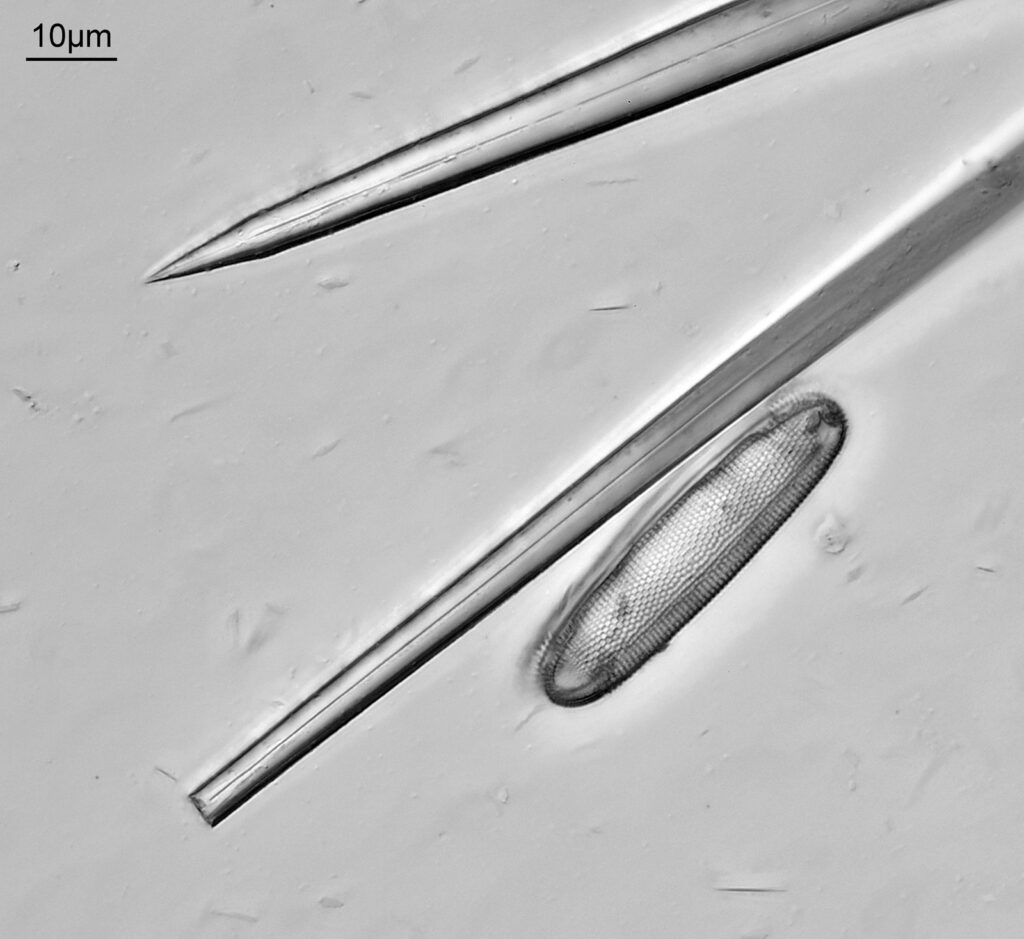
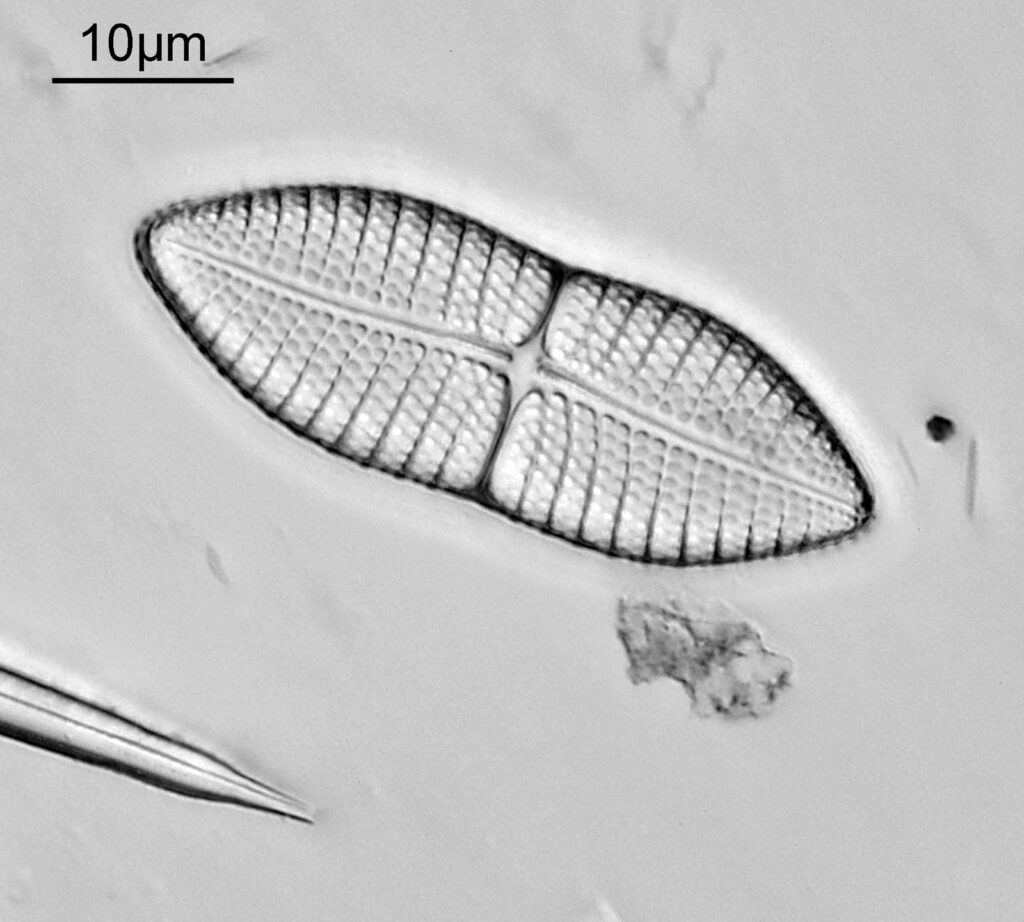
Bit of an update today on a new piece of equipment for my UV microscopy work. A few days ago I was at a Quekett Microscopical Club meeting, and one of my microscopy friends approached me and offered me something they had recently got as part of a consignment of microscope parts – a condenser made by Beck and described as ‘Quartz condenser, NA 1.25, W.I.’. I nearly fell off my seat, as quartz condensers are rather rare. Anyway I jumped at the chance to have it, and this post shares some initial images of it, and shows the transmission through it in the UV.
Here’s the condenser.


It came in a mount which is slightly too big for my Olympus BHB but the condenser itself unscrews from the mount and is RMS threaded as shown below.

Last year I had a condenser holder made which is RMS threaded and fits my microscope, so this will fit just fine. It also has a small diameter so will fit through the hole in my stage.
Why the excitement? Normal glass blocks UV, especially the shorter wavelength end of the UV spectrum below 365nm. As such it is no good for imaging at 313nm or below. Quartz however lets UV through and is good with light down to at least 250nm. Unfortunately quartz condensers were both rare and expensive when originally made, and as far as I am aware no-one currently makes them, which makes finding them a challenge. While I keep an eye out for them in the usual places, finding them is uncommon, and usually needs an element of luck (as was the case this time). This one has ‘W.I.’ written on the side, which means it is designed for use with a drop of water as the immersion fluid, although for low NA applications (when the objective is below NA 1) it would probably be fine to use it dry.
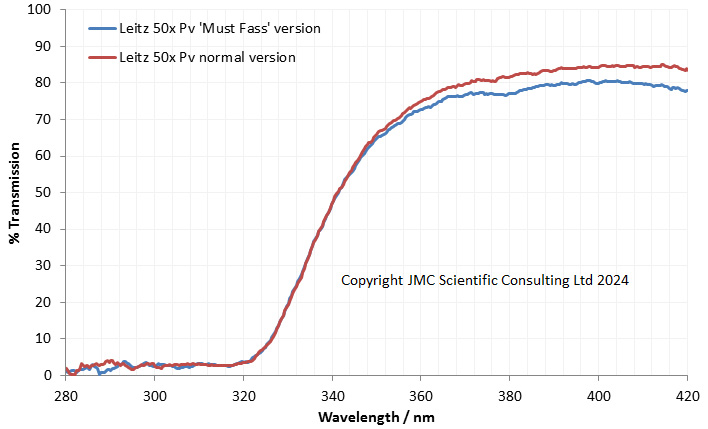
As I had not seen one like this before, the first thing I did was measure the transmission spectrum through it using my Ocean Insight FX spectrometer, and got the following.
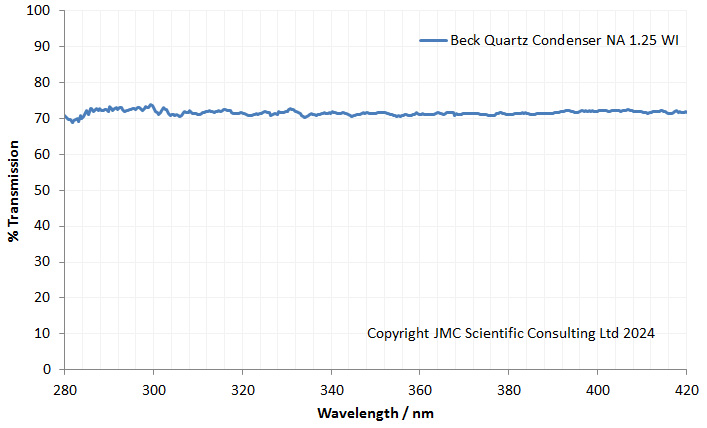
As expected, the transmission through it in the UV was good, not dropping at wavelengths below 365nm as would be expected for a normal glass one.
I have not seen one like this before, but I suspect it was originally designed for fluorescence work rather than UV microscopy (as mentioned for a couple of quartz condensers in this Beck catalogue) and is probably a simple Abbe construction. Simpler is normally better when it comes to UV.
Joining a club such as the Quekett is a great way to meet very knowledgeable and passionate folks, but also to find historically interesting items which you may otherwise never get to see, and I can well recommend it. I now look forward to trying this out for my UV microscopy work. As always, thanks for reading and if you’d like to know more about my work I can be reached here.