As it was Easter recently, I had a bit of spare time to do some microscopy. This time I returned to one of the diatom slides I had made using a quartz slide and coverslip so it could be used in short wavelength UV light. I thought this would be a good time to try out a trio of Leitz UV objectives (16x NA 0.25, 40x NA 0.65 and 100x NA 1.20) for imaging at 313nm. However as we shall see, things didn’t quit go to plan.
For the imaging I used my UV modified Olympus BHB microscope, and a monochrome converted Nikon d800 camera. Condenser was a Zeiss quartz one (simple brightfield illumination). Light source was a Zeiss mercury Xenon lamp. Edmund Optics 313nm, 10nm bandpass filters (2 stacked together) were used to filter the light going to the camera. The subject was a diatom slide which had a Gyrosigma (balticum, maybe) along with 2 other diatoms. Images have been reduced in overall size for sharing here.
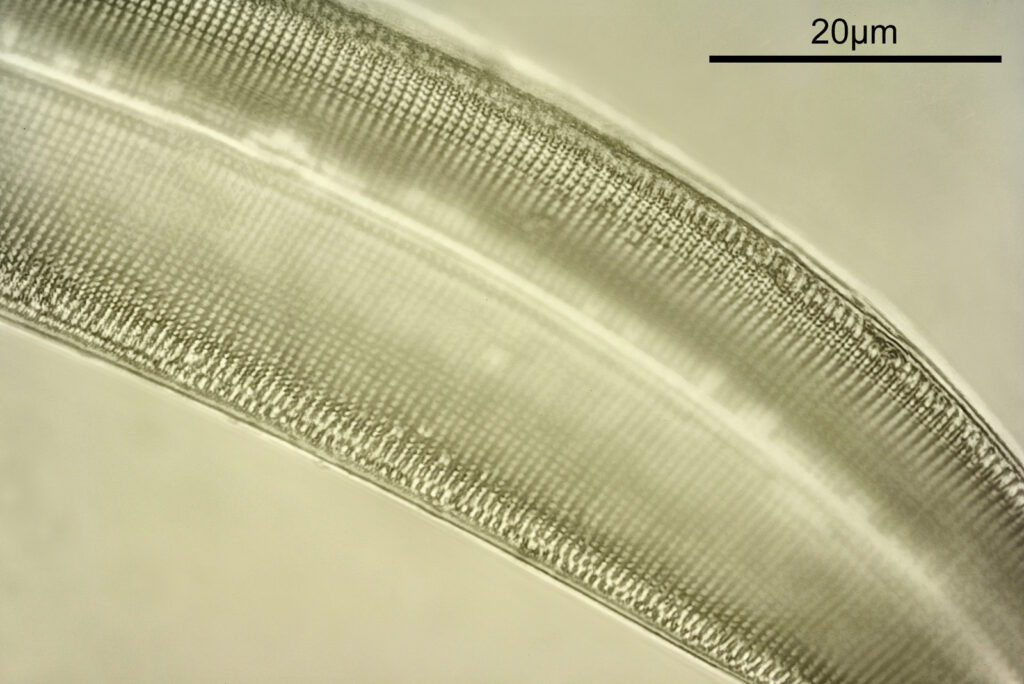
First, a stacked image using the 40x objective, showing part of the Gyrosigma (stacked using Zerene).

Plenty of detail to be seen with the 40x objective. Even being NA 0.65, the short wavelength light results in improved resolution compared with visible light imaging under the same conditions. As I had the system setup, I thought next I would try the 100x NA 1.20 objective (again as a stack). Unfortunately the depth of focus this objective has meant that I could not move the stage enough to get all of the diatom in focus, and the image only shows part of the structure in focus.
This highlights the issues with these high magnification, high NA objectives, i.e. very shallow focus depth. This make imaging thick samples a problem. Perhaps a thinner coverslip would work here, although likely with a bit of loss of image quality (more on that later). Final image, using the 16x objective, not stacked this time.

The 16x image shows the 3 diatoms on the slide. Speaking of the slide, this is it.

Here are the three objectives.


These objectives are interesting and I have been able to find out very little about them. They seem to be the Leitz equivalent of the Zeiss Ultrafluars and are very well made. The 40x and 100x are designed for glycerine immersion, and as I am writing this I have just had a thought. I use a 50:50 mix of glycerine and water for my immersion as this was what I got from a microscope supplier when I started out. This will have a different refractive index compared with pure glycerine. I wonder if this reduced the depth of focus for the 100x objective? I will park that idea and check later. The only mention I have found of ‘Leitz UV objectives’ is in a brochure from 1985 which I have shared before (here). Thing is in the brochure the coverslip thickness is quoted as 0.17mm, not the 0.35mm ones written on the objectives themselves. All very odd. Perhaps a thinner coverslip (0.20 or 0.25mm) might be an option and get me to image slightly thicker samples, although whether this would drop the resolution with the 100x objective would need to be checked.
A good friend asked me recently what was the actual benefit of these quartz based optics and UV light over conventional methods for improving resolution. Funnily enough I have written a bit about that before (here) and there isn’t much between say UV imaging at 313nm and imaging with blue light and a high NA objective and oblique illumination. I do it because I enjoy it and it was a scientific challenge to make the microscope. However where it will get more interesting is below 300nm, as the shorter the wavelength the greater the resolution. It is proving challenging that far into the UV though, and more work needs to be done. The microscopists of the early 1900s managed it though, so I know it can be done.
As always, thanks for reading, and if you’d like to know more about my work I can be reached here.
